Abstract
The cosmetic industry adapts to the needs of consumers seeking to limit the use of preservatives and develop of preservative-free or self-preserving cosmetics, where preservatives are replaced by raw materials of plant origin. The aim of study was a comparison of the antimicrobial activity of extracts (Matricaria chamomilla, Aloe vera, Calendula officinalis) and essential oils (Lavandulla officinallis,Melaleuca alternifolia, Cinnamomum zeylanicum) with methylparaben. Extracts (2.5 %), essential oils (2.5 %) and methylparaben (0.4 %) were tested against Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC25922,Staphylococcus aureus ATCC 29213, Candida albicans ATCC 14053. Essentials oils showed higher inhibitory activity against tested microorganism strain than extracts and methylparaben. Depending on tested microorganism strain, all tested extracts and essential oils show antimicrobial activity 0.8–1.7 and 1–3.5 times stronger than methylparaben, respectively. This shows that tested extracts and essential oils could replace use of methylparaben, at the same time giving a guarantee of microbiological purity of the cosmetic under its use and storage.
Keywords: Antimicrobial activity, Essential oils, Herbal extracts, Methylparaben
Introduction
Preservatives are added to cosmetics to maintain their microbiological purity during manufacture, packing, storage, but especially during the entire period of use. Despite the fact that preservatives are usually used in small concentrations, they are considered as one of the main factors causing allergies to users [1]. The main component of preservative system in most cosmetics product on the market is methylparaben which antimicrobial activity is well documented [2]. Methylparabens are more effective against fungi than against bacteria; their antibacterial activity is greatest against gram-positive organisms and poorest against Pseudomonas species [3]. Despite the numerous advantages of methylparaben as an effective preservative, some studies suggest that this component of cosmetic formula could pose some risks to human health [4, 5]. Therefore, there is a growing demand for cosmetics that are preservatives free. Alternative way to solve the problem of microbial purity of cosmetics is use of compounds (e.g. herbal extracts and essential oils) which are not preservatives but also exhibit antimicrobial activity. There are many studies carried out in the in vitro conditions, which evidence microbial activity of herbal extracts [6–8] and essential oils [9–11]. Therefore, it appears that these natural compounds can successfully be used in the cosmetic industry as a preservative. The aim of this study was a comparison of the antimicrobial activity of selected herbal extracts (Matricaria chamomilla, Aloe vera, Calendula officinalis) and essential oils (Lavandulla officinallis,Melaleuca alternifolia, Cinnamomum zeylanicum) to methylparaben in cosmetic emulsion.
Materials and Methods
Microorganisms
Pseudomonas aeruginosa ATCC 27853, Escherichia coli ATCC25922,Staphylococcus aureus ATCC 29213, Candida albicans ATCC 14053 were used. The microorganisms were activated through double passaging: bacteria on Trypticase Soy Agar medium (TSA; BioMerieux, France) (37 °C, 48 h) and yeast on Sabouraud Dextrose Agar medium (SDA; BioMerieux, France) (25 °C, 72 h).
Extracts and Essential Oils
Commercial available hydroglycolic extracts from Matricaria chamomilla flower, Aloe. vera leaf, Calendula officinalis flower (Greentech, France) and essential oils from Lavandulla officinallis,Melaleuca alternifolia, Cinnamomum zeylanicum (Avicenna-Oil, Poland) were used for the experiments.
Antimicrobial Activity of Essential Oils, Extracts and Methylparaben In Vitro
Several colonies of overnight cultures of individual organisms were suspended in saline to obtain density equal to 0.5 McFarland turbidity standard (approximate cell density of 1.5 × 108 CFU/mL). The antibacterial and antifungal activity was evaluated by using the disc diffusion method. Suspensions of microorganisms were spread over the TSA and SDA agar plates (BioMerieux, France), respectively by using sterile cotton swabs. The sterile paper discs of 6 mm in diameter (BTL, Poland) were impregnated with 15 μL essential oil/extracts/methylparaben and placed on the agar surface. Kanamycin (BioMerieux, France) and in the case of fungi amphotericin B (BioMerieux, France) were used as controls. All bacterial plates were incubated at 37 °C for 24 h and fungal plates at 25 °C for 72 h. The diameter of the zone of inhibition was measured in mm. For each test, three replicates were performed.
Preparation of Emulsion
The composition of formulations is showed in Table 1. Emulsifier Ceteareth-20 (Eumulgin® B2, Cognis Polska Sp. z.o.o.), Isopropyl Myristate (Cognis Polska Sp. z.o.o.), Ethylhexyl stearate (Cetiol® 868, Cognis Polska Sp. z.o.o.), Octyldecanol (Eutanol G®, Cognis Polska Sp. z.o.o.), Cyclopentasiloxane and Dimethicone (Dow Corning®1411 Fluid, Dow Corning Europe S.A.), water, glycerin (POCH S.A, Poland) and methylparaben (Aseptin M, Cognis Polska Sp. z.o.o.)/essential oil/extract were homogenized for 3 min using Heidolph SilentCrusher M homogenizer (Heidolph Instruments GmbH & Co. KG, Germany) at approximately 13,000 rpm. Then emulsion was gently stirred using blender RW 16 (IKA® Werke GmbH & Co. KG, Germany) and the Sodium Polyacrylate (Cosmedia SP, Cognis Polska Sp. z.o.o.) was added stepwise. Stirring continued for an additional 30 min. Extracts and essential oils were added at 2.5 % concentration. Methylparaben was added at 0.4 % concentration as the maximum allowable concentration of synthetic preservative specified by EU Cosmetics Directive. Emulsion without methylparaben, essential oils and extracts was a references sample.
Table 1.
Composition of the emulsions: E1, emulsion without methylparaben/essential oils/extracts; E2, emulsion with essential oils; E3, emulsion with extracts; E4, emulsion with methylparaben
| Ingredient* | Percentage by weight | |||
|---|---|---|---|---|
| E1 | E2 | E3 | E4 | |
| Sodium polyacrylate | 0.8 | 0.8 | 0.8 | 0.8 |
| Ceteareth-20 | 3 | 3 | 3 | 3 |
| Isopropyl myristate | 4 | 4 | 4 | 4 |
| Ethylhexyl stearate | 3 | 3 | 3 | 3 |
| Octylodecanol | 3 | 3 | 3 | 3 |
| Cyclopentasiloxane and dimethicone | 3 | 3 | 3 | 3 |
| Glycerin | 3 | 3 | 3 | 3 |
| Essential oil | 0 | 2.5 | 0 | 0 |
| Extract | 0 | 0 | 2.5 | 0 |
| Methylparaben | 0 | 0 | 0 | 0.4 |
| Aqua | 80.2 | 77.7 | 77.7 | 79.8 |
*INCI Name
Antimicrobial Effectiveness Testing
The suspensions of the tested bacteria and yeast cells were prepared in saline and later standardized to the density of about 108 CFU/mL. Inoculation of emulsion (with/without selected extracts, essential oils and methylparaben) with the microorganism suspension was prepared by addition of 0.02 mL inoculum to 20 g cream sample. The inoculated containers were mixed thoroughly and incubated in the dark at 20–25 °C. The number of viable microorganism in formulations was determined by the plate count method at the proper times 0, 7, 14, 21, and 28 days after inoculation. A sample of 1 mL cream was transferred to 9 mL physiological salt solution (0.9 % NaCl Graso Biotech) and tenfold dilutions method was done. Triplicate plating of each dilution was performed with TSA agar for bacteria and SDA agar for yeast. The plates were incubated at 37 and 25 °C for bacteria and yeast respectively. Counts the CFU per plate (30–300 colonies) determined the number of surviving microorganisms per gram of tested cosmetic product. The results were expressed as log CFU/mL. All determinations were performed in triplicate, and the results represent an average of two different experiments.
Statistical Analysis
All tests were conducted in triplicate and data from experiments were calculated as mean ± SD. Standard deviation for the test of microorganism population viability not exceeding 0.3 logarithmic unit.
Results
Antimicrobial Activity of Essential Oils, Extracts and Methylparaben In Vitro
Antimicrobial activity of the extracts and essential oils was checked in the disc-diffusion test and presented in Table 2. Essentials oils in the examined concentrations showed higher inhibitory activity against bacteria and yeast than extracts and methylparaben. Depending on of tested microorganism strain, the zone of inhibition varied between 8–44 mm for essential oils, 7–9 mm for extracts and 8–9 mm for methylparaben. Lavender oil and Calendula officinalis extract did not affect the growth of P. aeruginosa. All tested extracts did not affect the growth of C. albicans. The ranking of the inhibitory activity of the essential oils, extracts and methylparaben determined on the basis of inhibition zone diameter was as follows: Cinnamomum zeylanicum > Lavandulla officinallis,Melaleuca alternifolia > methylaparaben > Aloe vera > Matricaria chamomilla, Calendula officinalis. The sensitivity of the microorganisms was as follows: S. aureus > C. albicans > E. coli > P. aeruginosa.
Table 2.
Antibacterial and antifungal activity of essential oils, extracts and methylparaben (15 μL) in agar disc diffusion method, inhibition zones (mm). Diameter of inhibition zones (mm) including the diameter of disc (6 mm), values are given as mean ± SD of triplicate experiment
| S. aureus | P. aeruginosa | E. coli | C. albicans | |
|---|---|---|---|---|
| Lavandulla officinallis | 17 ± 0.66 | – | 18 ± 0.4 | 15 ± 2.0 |
| Melaleuca alternifolia | 17 ± 3.33 | 8 ± 0.44 | 11 ± 0.66 | 10 ± 4.44 |
| Cinnamomum ceylanicum | 44 ± 2.66 | 24 ± 2.22 | 32 ± 1.33 | 39 ± 1.77 |
| Matricaria chamomilla extract | 7 ± 0.66 | 7 ± 0.44 | 7 ± 0.66 | – |
| Aloe vera extract | 7 ± 0.44 | 9 ± 0.44 | 8 ± 0.44 | – |
| Calendula officinalis extract | 8 ± 1.1 | – | 7 ± 1.1 | – |
| Methylparaben (0.4 %) | 9 ± 0.44 | 8 ± 0.44 | 8 ± 0.44 | 9 ± 0.44 |
Antimicrobial Effectiveness Testing
It was found that essentials oils showed higher inhibitory activity against bacteria and yeast than extracts and methylparaben (Figs. 1, 2). Among all tested compounds, cinnamon oil was the most potent inhibitor of microorganism growth. This essential oil completely inhibited S. aureus, E. coli and C. albicans growth after 28 days of incubation. Tea tree oil, extracts from Calendula officinallis and Matricaria chamomilla totally inhibited E. coli growth, while Aloe vera extract overall stopped S. aureus growth after 28 days of incubation. For all tested creams with extracts, essential oils or methylparaben, the weakest antimicrobial activity was demonstrated against P. aeruginosa. All tested extracts and essential oils at 2.5 % concentration exhibited antimicrobial activity stronger than methylparaben. Depending on tested microorganism strain, extracts and essential oils showed antimicrobial activity 0.8–1.7 and 1–3.5 times stronger than methylparaben, respectively.
Fig. 1.
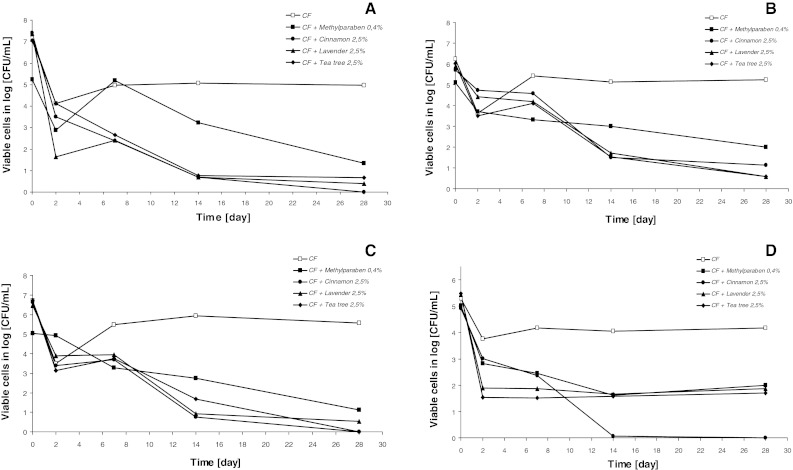
Inhibition of growth of Staphylococcus aureus ATCC 29213 (a), Pseudomonas aeruginosa ATCC 27853 (b), Escherichia coli ATCC 25922 (c), Candida albicans ATCC 14053 (d) in cream formulation (CF) with Cinnamomum zeylanicum (cinnamon), Lavandulla officinallis (lavender), Melaleuca alternifolia (tea tree) oils and methylparaben
Fig. 2.
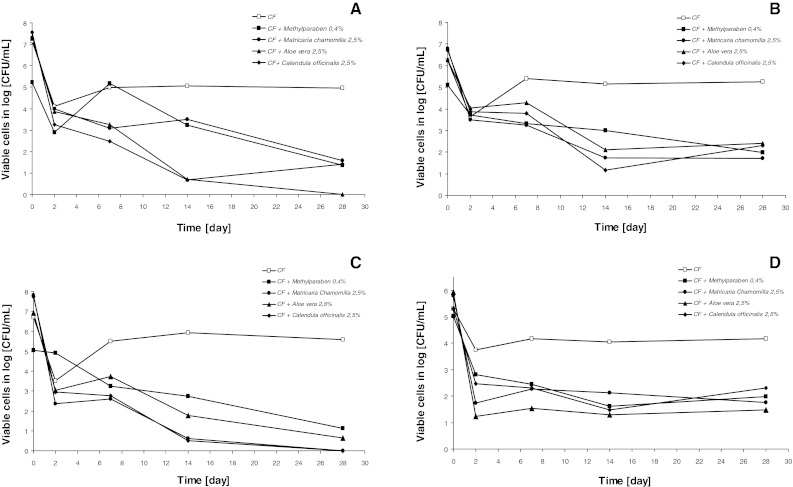
Inhibition of growth of Staphylococcus aureus ATCC 29213 (a), Pseudomonas aeruginosa ATCC 27853 (b), Escherichia coli ATCC 25922 (c), Candida albicans ATCC 14053 (d) in cream formulation (CF) with Matricaria chamomilla, Aloe vera, Calendula officinalis extracts and methylparaben
Discussion
Antimicrobial activity of essential oils and herbal extracts in in vitro conditions is well documented. In our study essentials oils revealed higher inhibitory activity both to bacteria and yeast compared to extracts and methylparaben. Lavender and cinnamon oils exhibited approximately 1–2 times higher activity against S. aureus and E. coli in comparison with results of others [12, 13]. We showed that tea tree oil inhibited the growth of all studied microbes; however its action against P. aeruginosa was weak. These results fully support the previously published studies, which demonstrated the resistance of P. aeruginosa on action of tea tree oil [8, 9]. In our research, it was found that Matricaria chamomilla and Aloe vera extracts effected growth of all tested strain. Obtained results were within the range previously described in the studies of other [7, 14, 15], where the inhibitory effects of Matricaria chamomilla and Aloe vera extracts against S. aureus, P. aeruginosa, E. coli and C. albicans were also determined. Only Calendula officinalis showed lower activity against P. aeruginosa, S. aureus, C. albicans than those observed by Roopashree et al. [16]. Determined antimicrobial activity of essential oils and extracts in cosmetic formulation was substantially lower than in in vitro condition. This observation fully supports results of previous studies [10, 17, 18]. It may result from interaction of essential oils and extracts with chemical components of cosmetic formulation. Higher affinity of essential oil/extracts for the oil/water cosmetic ingredients limits their accessibility in the water/oil phase resulting in antimicrobial activity reduction. Therefore, the results of in vitro studies are not enough to confirm or deny the antimicrobial properties of natural compounds in the cosmetic formulation. It is necessary to carried out the experiments that take into account the possible interactions between the additive of natural origin and other cosmetic ingredients.
In our research, essential oils and extracts at the concentration of 2.5 % inhibited growth of all tested microorganism strain. This supports previous published data show that most essential oils exhibit antimicrobial activity at the concentration below 5 % (v/v) [19]. Moreover, the antimicrobial activity of oils is dose-dependent with greater activity seen at the higher oil concentrations. The study concerning the antimicrobial effect of tea tree oil showed that hygienic skin wash with 5 % tea tree oil was significantly more active against E. coli K12 growth than a non-medicated soft soap [20]. Manou et al. [17] found that 3 % Thymus vulgaris essential oil inhibited the growth of S. aureus, P. aeruginosa and E. coli in formulations O/W and W/O, C. albicans only in formulations W/O, but not against the A. niger. The 1 and 2 % (v/v) Calamintha officinalis essential oil added to two product types O/W cream and shampoo also inhibited growth of tested bacteria and fungi alone and as mixed culture [18]. Lavandulla officinallis and Rosmarinus officinalis oils (1.5 %) in an aqueous cream formulation displayed remarkable antimicrobial activities against all common test organisms (including bacteria and fungi) and environmental isolates used [21]. Mixture of different extracts and essential oils can very effectively inhibit the growth of microorganisms at a much broader spectrum of activity and replace or significantly reduce the amount of synthetic preservatives added to cosmetics. It is known that antimicrobial activity of essential oils is much weaker than synthetic preservatives [22] and for this reason they must be added in greater amounts to the cosmetic. We found that adding to the cream formulation five times more essential oils and extracts (2.5 % v/v) compared with methylparaben (0.4 % v/v) resulted in a stronger activity against tested microorganisms even against most resistant P. aeruginosa. Kunicka-Styczyńska et al. [23] showed that in washing liquid, 1 % tea tree oil, 1 % lavender oil or their mixture containing 0.5 % each is sufficient to inhibit most of the microorganisms tested. At the same time, the supplementation of oil mixtures with a small amount of a commercial synthetic preservative—Glydant Plus Liquid (0.1 %) eliminated even the most resistant microorganism—P. aeruginosa and A. niger from the formulation. The creation of such mixture makes it possible to decrease the dose of the synthetic preservative by 8.5 times compare to the dose recommended by the producer. Synergism between essentials oils and methylparaben was observed by Maccioni et al. [24]. They found that Laurus nobilis, Eucalyptus globulus and Salvia officialis oils added at concentration of 0.025 and 0.0125 % were 200-fold more active in combination with methylparaben. Also Patrone et al. [25] showed synergism between eucalyptus and mint oils with methylparaben against P. aeruginosa and mint, oregano and sage combined with propylparaben and imidazolidinyl urea against S. aureus. It must be stressed that the use of high concentrations of essential oils in formulation exclude too intense fragrance of cosmetics and can cause a skin irritation and allergies to users. Less irritancy occur at lower oil concentrations, but at the same time, low concentration of essential oils reduces their antibacterial activity and usability as preservatives. These significant disadvantages of essential oils decrease their use as preservatives in the cosmetic industry. Therefore, in the present study we also examined the antimicrobial properties of plant extract which show lower irritant properties than essential oils. Bernatoniene et al. [26] described that 0.9 % Calendula officinalis extract added to cream inhibited growth of P. aeruginosa and S. aureus. They showed that no more than 104 microorganisms (aerobic bacteria plus fungi) per 1 g of cream was found in samples collected directly after preparation and after 12 months of storage. This work proved that the antimicrobial efficiency of Calendula officinalis extract was sufficient to preserve the formulation against microorganism contamination. Papageorgiou et al. [27] found that Lonicera caprifolium and Lonicera japonica extracts added to different aqueous (tonic lotion, shampoo, shower gel) and O/W formulation (conditioning cream, anticellulite cream, cleansing milk, peeling cream) at the concentration of 0.2 % have excellent activity against S. aureus, P. aeruginosa, E. coli, C. albicans, A. niger in the acidic pH 5.5 during 28 day incubation and following 3 weeks of use. Effectiveness of antimicrobial protection of Rubus rosaefolius Smith extract 0.2 % (w/w) in two different base formulations (emulsion and gel) was investigated by Ostrovsky et al. [28]. In both examined emulsions, extract was effective against P. aeruginosa, B. cepacia, S. aureus and E. coli, presenting a lethal effect at 2 days after inoculation and maintained for 28 days of incubation. In other studies, the preservative efficacy of chitosan and Inula helenium was evaluated [29]. The mixture of chitosan powder and Inula helenium extract powder in the ratio 1:3 was incorporated at concentrations of 5–10 % (w/w) in O/W emulsion formulas and tonic lotion. It was found that this mixture showed antimicrobial activity against S. aureus, P. aeruginosa, C. albicans, A. niger and E. coli in the O/W formulas and the tonic. However, despite the many advantages of using natural extracts as antimicrobial additives in the cosmetic, the use of plant extracts might be accompanied by various other problems including change of color, odor and emulsion stability [30].
Summarizing, examined extracts and essential oils inhibit the growth of microorganisms more firmly than methylparaben. Therefore they can be recommended as effective candidate for natural cosmetic preservatives. Addition to cosmetic formula the appropriate concentrations and mixtures of essential oils and extracts may let for replace the synthetic preservatives as methylparaben, at the same time giving a guarantee of microbiological purity of the cosmetic product under its use and storage.
References
- 1.Wong S, Street D, Delgado SI, Klontz KC. Recalls of food and cosmetics due to microbial contamination reported to the U.S. Food and Drug Administration. J Food Protect. 2000;63:1113–1116. doi: 10.4315/0362-028x-63.8.1113. [DOI] [PubMed] [Google Scholar]
- 2.Andersen FA. Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol. 2008;27:1–82. doi: 10.1080/10915810802548359. [DOI] [PubMed] [Google Scholar]
- 3.Rietschel RL, Fowler JF. Allergy to preservatives and vehicles in cosmetics and toiletries. In: Rietschel RL, Fowler JF, editors. Fisher’s contact dermatitis. 5. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 266–319. [Google Scholar]
- 4.Okamoto Y, Hayashi T, Matsunami S, Ueda K, Kojima N. Combined activation of methyl paraben by light irradiation and esterase metabolism toward oxidative DNA damage. Chem Res Toxicol. 2008;21:1594–1599. doi: 10.1021/tx800066u. [DOI] [PubMed] [Google Scholar]
- 5.Handa O, Kokura S, Adachi S, et al. Methylparaben potentiates UV-induced damage of skin keratinocytes. Toxicology. 2006;227:62–72. doi: 10.1016/j.tox.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Bisht SPS, Mishra R, Kumari K. Antimicrobial and free radical scavenging activity of Chammomile flower essential oil. Asian J Pharm Health Sci. 2011;1:283–285. [Google Scholar]
- 7.Thiruppathi S, Ramasubramanian V, Sivakumar T, Thirumalaiarasu V. Antimicrobial activity of Aloe vera (L.) Burm. f. against pathogenic microorganisms. J Biosci Res. 2010;1:251–258. [Google Scholar]
- 8.Hammer KA, Garson CF, Rile TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86:985–990. doi: 10.1046/j.1365-2672.1999.00780.x. [DOI] [PubMed] [Google Scholar]
- 9.Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunicka-Styczyńska A, Sikora M, Kalemba D. Antimicrobial activity of lavender, tea tree and lemon oils in cosmetic preservative systems. J Appl Microbiol. 2009;107:1903–1911. doi: 10.1111/j.1365-2672.2009.04372.x. [DOI] [PubMed] [Google Scholar]
- 11.Sandigawad BM, Patil CG. The in vitro antibacterial activity of cinnamomum species A. Asian J Exp Biol Sci. 2010;1:434–439. [Google Scholar]
- 12.Pandey A, Jagtap JV, Polshettiwar SA. Formulation and evaluation of in vitro antimicrobial activity of gel containing essential oils and effect of polymer on their antimicrobial activity. Int J Pharm Pharm Sci. 2011;3:234–237. [Google Scholar]
- 13.Serban ES, Ionescu M, Matinca D, Maier CS, Bojita MT. Screening of the antibacterial and antifungal activity of eight volatile essential oils. Farmacia. 2011;59:440–446. [Google Scholar]
- 14.Ababutain IM. Antimicrobial activity of ethanolic extracts from some medicinal plant. Aust J Basic & Appl Sci. 2011;5:678–683. [Google Scholar]
- 15.Grover A, Bhandari BS, Rai N. Antimicrobial activity of medicinal plants-Azadirachta indica A. Juss, Allium cepa L. and Aloe vera L. Int J Pharm Tech Res. 2011;3:1059–1065. [Google Scholar]
- 16.Roopashree TS, Dang R, Rani S, Narendra C. Antibacterial activity of antipsoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. Int J Appl Res Nat Prod. 2008;1:20–28. [Google Scholar]
- 17.Manou I, Bouillard L, Devleeschouwer MJ, Barel AO. Evaluation of the preservative properties of Thymus vulgaris essential oil in topically applied formulations under a challenge test. J Appl Microbiol. 1998;84:368–376. doi: 10.1046/j.1365-2672.1998.00353.x. [DOI] [PubMed] [Google Scholar]
- 18.Nostro A, Cannatelli MA, Morelli I, Musolino AD, Scuredi F, Pizzimenti F, Alonzo V. Efficiency of Calamintha officinalis essential oil as preservative in two topical product types. J Appl Microbiol. 2004;97:395–401. doi: 10.1111/j.1365-2672.2004.02319.x. [DOI] [PubMed] [Google Scholar]
- 19.Thormar H (2011) Antibacterial and antifungal activities of essential oils. In: Lipids and essential oils as antimicrobial agents. Wiley, Chichester, pp 256–293
- 20.Messager S, Hammer KA, Carson CF, Riley TV. Effectiveness of hand-cleansing formulations containing tea tree oil assessed ex vivo on human skin and in vivo with volunteers using European standard EN 1499. J Hosp Infect. 2005;59:220–228. doi: 10.1016/j.jhin.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 21.Muyima NYO, Zulu G, Bhengu T, Popplewell D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr J. 2001;17:258–266. doi: 10.1002/ffj.1093. [DOI] [Google Scholar]
- 22.Kabara JJ. Aroma preservatives, essential oils and fragrances as antimicrobial agents. In: Kabara JJ, editor. Cosmetics and drug preservation principles and practice. New York: Basel Marcel Dekker, Inc.; 1984. pp. 237–273. [Google Scholar]
- 23.Kunicka-Styczyńska A, Sikora M, Kalemba D. Lavender, tea tree and lemon oils as antimicrobials in washing liquids and soft body balms. Int J Cosmet Sci. 2011;33:53–61. doi: 10.1111/j.1468-2494.2010.00582.x. [DOI] [PubMed] [Google Scholar]
- 24.Maccioni AM, Anchisi C, Sanna A, Sardu C, Dessi S. Preservative systems containing essential oils in cosmetic products. Int J Cosmet Sci. 2001;24:53–59. doi: 10.1046/j.0412-5463.2001.00113.x. [DOI] [PubMed] [Google Scholar]
- 25.Patrone V, Campana R, Vittoria E, Baffone W. In vitro synergistic activities of essential oils and surfactants in combination with cosmetic preservatives against Pseudomonas aeruginosa and Staphylococcus aureus. Curr Microbiol. 2010;60:237–241. doi: 10.1007/s00284-009-9531-7. [DOI] [PubMed] [Google Scholar]
- 26.Bernatoniene J, Masteikova R, Davalgiene J, et al. Topical application of Calendula officinalis (L.): formulation and evaluation of hydrophilic cream with antioxidant activity. J Med Plant Res. 2011;5:868–877. [Google Scholar]
- 27.Papageorgiou S, Varvaresou A, Tsirivas E, Demetzos C. New alternatives to cosmetics preservation. J Cosmet Sci. 2010;61:107–123. [PubMed] [Google Scholar]
- 28.Ostrosky EA, Marcondes EM, Nishikawa Sde O, et al. Rubus rosaefolius extract as a natural preservative candidate in topical formulations. AAPS PharmSciTech. 2011;12:732–737. doi: 10.1208/s12249-011-9635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo SB, Ryu CS, Ahn GW, et al. Development of a natural preservative system using the mixture of chitosan-Inula helenium L. extract. Int J Cosmet Sci. 2002;24:195–206. doi: 10.1046/j.1467-2494.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 30.Varvaresou A, Papageorgiou S, Tsirivas E, Protopapa E, Kintziou H, Kefala V, Demetzos C. Self-preserving cosmetics. Int J Cosmet Sci. 2009;31:163–175. doi: 10.1111/j.1468-2494.2009.00492.x. [DOI] [PubMed] [Google Scholar]


