Figure 1.
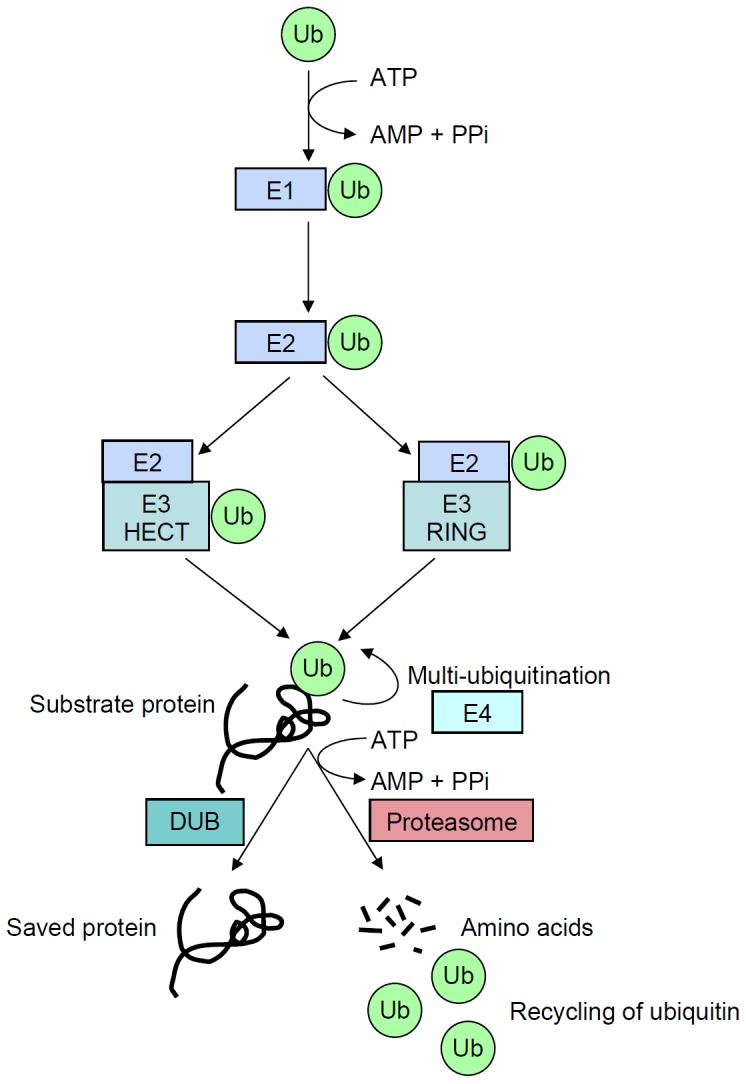
Schematic representation of the ubiquitination process that target proteins for degradation. A ubiquitin molecule (Ub) is first linked by its carboxy-terminal amino acid to an E1-activating enzyme (E1) through a high-energy bond on a cysteine residue, while consuming energy (ATP). Activated ubiquitin is then translocated to the E2-conjugating enzyme (E2). RING E3 ubiquitin ligases (E3 RING) catalyze the transfer of ubiquitin directly from E2 to the substrate, whereas HECT E3 enzymes (E3 HECT) accept activated ubiquitin from E2 before transferring it to the substrate. Following this first step, called monoubiquitination, the process may be repeated by some processive E2/E3 enzymes, or with the help of E4 enzymes and, finally, leads to polyubiquitination. Proteins targeted for degradation into amino acids can however be rescued by deubiquitining enzymes (DUB).
