Figure 5.
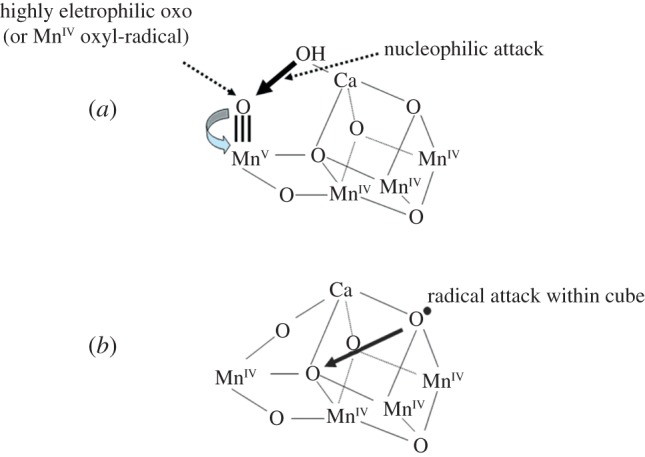
Two different mechanisms for the final step of the S-state cycle when the dioxygen bond of O2 is formed. (a) Mechanism 1. The very high oxidation state of the Mn-cluster, particularly the Mn ion outside the Mn3CaO4-cubane, leads to a high electron deficient oxo (after deprotonation of water molecules during the S-state cycle). Nucleophilic attack by the hydroxide of the second substrate water within the coordination sphere of Ca2+ leads to O2 formation. (b) Mechanism 2. The formation of an oxo-radical within the Mn3CaO4-cubane attacks a bridging oxo species to form the O–O bond. (Online version in colour.)
