Abstract
Background
Neutrophil elastase plays a crucial role in the development of acute lung injury (ALI) in patients with systemic inflammatory response syndrome (SIRS). The clinical efficacy of the neutrophil elastase inhibitor, sivelestat, for patients with ALI associated with SIRS has not been convincingly demonstrated. The aim of this study was to determine if there are clinical features of patients with this condition that affect the efficacy of sivelestat.
Methods
This was a retrospective study of 110 ALI patients with SIRS. Clinical information, including the etiology of ALI, the number of organs failing, scoring systems for assessing the severity of illness, and laboratory data, was collected at the time of diagnosis. Information on the number of ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after the time of ALI diagnosis was also collected. The effect of sivelestat on ALI patients was also examined based on whether they had sepsis and whether their initial serum procalcitonin level was ≥0.5 ng/mL.
Results
There were 70 patients who were treated with sivelestat and 40 control patients. VFDs and ΔP/F were significantly higher in the treated patients than in the control patients. However, there was no significant difference in the patient survival rate between the two groups. Sivelestat was more effective in ALI patients with a PaO2/FIO2 ratio ≥ 140 mmHg or sepsis. Sivelestat significantly prolonged survival and led to higher VFDs and increased ΔP/F in septic patients and patients with initial serum procalcitonin levels ≥ 0.5 ng/mL.
Conclusion
The results may facilitate a future randomized controlled trial to determine whether sivelestat is beneficial for ALI patients with sepsis.
Keywords: systemic inflammatory response syndrome, procalcitonin, ventilator-free days, neutrophil elastase
Introduction
Acute lung injury (ALI), with acute onset, bilateral pulmonary infiltrates, and hypoxemia, is a complex disorder characterized by pulmonary inflammation and increased pulmonary vascular permeability.1 ALI is caused by an excessive inflammatory response to various assaults on the body, such as pneumonia, sepsis, trauma, and surgery. The pathogenesis of ALI involves inflammatory reactions associated with the accumulation of neutrophils in the lungs.2–6 In particular, elastase, which is released from activated neutrophils, has attracted attention as a factor that causes lung injury in patients with systemic inflammatory response syndrome (SIRS).7
Systemic inflammation induces vascular endothelial injury and results in organ dysfunction.8,9 Leukocyte–endothelial cell interaction resulting from systemic inflammation plays an important role in the pathogenesis of vascular endothelial injury.8,9 Neutrophil elastase, located downstream in the humoral mediator network, contributes to the development of vascular endothelial injury in concert with other mediators, which leads to increased permeability, vasodilation, and activation of the coagulation cascade.10
Sivelestat (Ono Pharmaceutical, Osaka, Japan) is a selective neutrophil elastase inhibitor11 that has been reported to be effective for endotoxin-induced lung injury in hamsters, guinea pigs, and sheep.11,12 However, the clinical efficacy of sivelestat in patients with SIRS and ALI remains controversial. Two clinical studies have shown that sivelestat reduced the duration of mechanical ventilation, shortened stays in the intensive care unit (ICU), and prolonged survival in patients with ALI,1,13 whereas the Sivelestat Trial in ALI Patients Requiring Mechanical Ventilation (STRIVE) study failed to demonstrate its efficacy.14 These discrepant results may be due to differences in patient characteristics such as age, baseline respiratory condition, and the number of non-pulmonary failed organs.1,14 In addition, there have been a few reports indicating that sivelestat is effective in patients with ALI or acute respiratory distress syndrome (ARDS) and sepsis.15,16 However, the clinical characteristics associated with the efficacy of sivelestat in patients with ALI associated with SIRS have not been convincingly elucidated.
The four aims of this study were to: (1) evaluate the clinical efficacy of sivelestat in ALI patients with SIRS, (2) determine if there are clinical features of patients with this condition that affect the efficacy of sivelestat, (3) assess the efficacy of sivelestat in ALI patients based on whether they have sepsis, and (4) assess the efficacy of sivelestat based on the initial serum procalcitonin (PCT) level.
Materials and methods
Study population
This was a retrospective study of ALI patients with SIRS who were admitted to Ehime University Hospital, Sumitomo Besshi Hospital, Ehime Prefectural Central Hospital, or Matsuyama Red Cross Hospital during the period 2009–2011.
Patients were excluded if they were aged < 20 years, if they had a neuromuscular disease that impaired spontaneous ventilation, severe chronic pulmonary disease, severe central nervous system disease, uncontrolled malignancy, or severe chronic liver disease. The ethical committee of each hospital approved the study protocol, and informed consent was waived because of the retrospective design.
Diagnosis of SIRS, ALI, disseminated intravascular coagulation (DIC), and sepsis
The diagnosis of SIRS was confirmed by the presence of at least two of the following (originally proposed by the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference17): body temperature <36°C or >38°C; heart rate > 90 bpm; respiratory rate > 20 breaths/minute or PaCO2 < 32 mmHg; white blood cell (WBC) count > 12,000 cells/μL or < 4000 cells/μL, or >10% immature (band) cells. The three criteria for the diagnosis of ALI, which were based on the definition from the American-European Consensus Conference,18 were: (1) PaO2/FIO2 (P/F) ≤ 300 mmHg, (2) bilateral pulmonary infiltrates on chest X-ray, and (3) pulmonary edema of non-cardiogenic origin (pulmonary capillary wedge pressure ≤ 18 mmHg or, in the absence of pulmonary capillary wedge pressure measurement, no clinical evidence of elevated left arterial pressure). The scoring system of the Japanese Association for Acute Medicine19 was used for the diagnosis of DIC (Table 1). The Japanese Association for Acute Medicine DIC score has been shown to identify most of the patients diagnosed by the overt International Society of Thrombosis and Hemostasis criteria.20
Table 1.
New scoring system for disseminated intravascular coagulation (DIC) by the Japanese Association for Acute Medicine19
| Criterion | Score |
|---|---|
| Systemic inflammatory response syndrome criteria | |
| ≥3 | 1 |
| 0–2 | 0 |
| Platelet count (× 109/L) | |
| <80 or >50% decrease within 24 hours | 3 |
| ≥80 and <120 or >30% decrease within 24 hours | 1 |
| ≥120 | 0 |
| Prothrombin time (value of patient/normal value) | |
| ≥1.2 | 1 |
| <1.2 | 0 |
| Fibrin/fibrinogen degradation products (mg/L) | |
| ≥25 | 3 |
| ≥10 and <25 | 1 |
| <10 | 0 |
Note: A score of four points or more is considered to indicate DIC.
Sepsis was diagnosed based on the criteria proposed by the Society of Critical Care Medicine/European Society of Intensive Care Medicine/American College of Chest Physicians/American Thoracic Society/Surgical Infection Society International Sepsis Definition Conference:21 confirmed source of infection and fulfillment of SIRS criteria. If these criteria were not fulfilled, patients were diagnosed as non-sepsis.
Intervention and treatment
All patients received pressure-controlled and pressure-supported mechanical ventilation with a positive end-expiratory pressure. At the time of ALI diagnosis, sivelestat was administered intravenously at a rate of 0.2 mg/kg/h continuously for a maximum of 14 days.
Data collection
Baseline data were collected from patient records. Clinical data, including the etiology of ALI, the number of failed organs, and the values of assessment systems (which included the sequential organ failure assessment [SOFA] score, the gas exchange, organ failure, cause, associated disease [GOCA] score, the SIRS score, and the DIC score), and the types of infections and causative pathogens, were collected at the time ALI was diagnosed. SOFA comprises separate scores for the respiratory, cardiovascular, renal, central nervous systems and coagulation and hepatic failure and each organ system may be awarded 0–4 points. The SOFA score can help assess organ dysfunction or failure over time and is useful to evaluate morbidity.22 The GOCA score is the sum of four variables: (1) the severity of gas exchange (0–3), (2) the number of failed organs (0–3), (3) the cause of lung injury (0: lung only, 1: direct lung injury, 2: indirect injury), and (4) associated diseases (0: no associated disease that will cause death within 5 years, 1: coexisting diseases that will cause death within 5 years but not within 6 months, 2: coexisting diseases that will cause death within 6 months). It was reported that the GOCA score might prove more convenient to use than the Acute Physiology and Chronic Health Evaluation (APACHE) II and the Simplified Acute Physiology Score (SAPS) II because it requires fewer variables but provides the same predictive power.23
Laboratory data, including WBC count, C-reactive protein level, and PCT level, were collected at the time ALI was diagnosed. For PCT measurements, we used a solid-phase, semi-quantitative immunoassay BRAHMS PCT-Q (Thermo Fisher Scientific Clinical Diagnostics BRAHMS GmbH, Hennigsdorf, Germany).
Sivelestat efficacy was evaluated based on survival rate, the number of ventilator-free days (VFDs) and change in PaO2/FIO2 ratio (ΔP/F) between the P/F values determined before and 7 days after diagnosis of ALI. “VFDs” were defined as the number of days (from day 1 to day 28) that a patient breathed without assistance.24
Statistical analysis
Results are expressed as median values, with interquartile ranges in parentheses. The Mann–Whitney U test or the chi-square test was used to compare groups. To assess the clinical efficacy of sivelestat, survival was analyzed using the Cox proportional hazards model with sex, age, P/F at the time of ALI diagnosis, the number of failed organs, septic status, and ALI etiology as covariates.
Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cut-off values for the P/F at the time of diagnosis that discriminated between survivors and non-survivors. A cut-off value for P/F that provided the highest sensitivity and specificity was chosen.
In this study, we used a solid-phase, semi-quantitative immunoassay for PCT measurements. This test kit categorized the PCT levels into four grades: (1) <0.5 ng/mL; (2) 0.5 to <2.0 ng/mL; (3) 2.0 to <10.0 ng/mL; and (4) ≥10.0 ng/mL. When the cut-off level was set as 0.5, 2.0, or 10.0 ng/mL, the sensitivity of PCT for sepsis was 95.9%, 59.5%, and 35.1%, the specificity was 86.1%, 91.7%, and 97.2%, and the diagnostic accuracy was 92.7%, 70.0%, and 55.5%, respectively. The PCT level at 0.5 ng/mL was the best cut-off value for PCT that could discriminate between septic and non-septic patients. In addition, previous reports demonstrated that the cut-off value for PCT was 0.5 ng/mL in differentiating between bacterial infection and other kinds of inflammatory processes.25 Therefore, in this study, we set the cut-off value for PCT at 0.5 ng/mL, and PCT levels ≥ 0.5 ng/mL were considered PCT positive.
When patients were divided into two groups – those with and those without sepsis, and those who were PCT positive and those who were PCT negative – a univariate analysis using the Cox proportional hazards model was used to assess the relationship between mortality and the following variables: sex; age; ALI etiology; the number of failed organs; administration of sivelestat; administration of steroids; SOFA, GOCA, SIRS, and DIC scores; P/F; WBC count; and C-reactive protein serum level at the time of ALI diagnosis. Parameters found to be significant by univariate analysis were taken as potential predictors of mortality and used as covariates in multivariate analysis to identify independent predictors of mortality. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated for the variables. The Kaplan–Meier method was used to estimate survival rates, and comparisons were made using the log-rank test. All tests were two-tailed, and P values < 0.05 were considered statistically significant. Statistical analysis was performed using SPSS for Windows version 19 (IBM, Armonk, NY, USA).
Results
Patient characteristics
A total of 537 ALI patients were enrolled in this study. Of these, we excluded 39 patients who were aged < 20 years, 147 patients with uncontrolled malignancy, 35 patients with severe chronic pulmonary diseases, 48 patients with severe chronic liver disease, three patients with neuromuscular disease that impaired spontaneous ventilation, 31 patients with severe central nervous system disease, 68 patients who did not receive mechanical ventilation, and 56 patients with inadequate data. This left 110 patients (71 men and 39 women) who were included in the study. The characteristics of the patients with SIRS at the time of ALI diagnosis are presented in Table 2. At baseline, there were no statistically significant differences between the patients treated with sivelestat (n = 70) and the control patients (n = 40).
Table 2.
Characteristics of systemic inflammatory response syndrome (SIRS) patients at the time of acute lung injury (ALI) diagnosis
| Characteristic | Control | Sivelestat | P-value |
|---|---|---|---|
| Patients | 40 | 70 | |
| Sex (male/female) | 23/17 | 48/22 | 0.243 |
| Age (yrs) | 71 (60–77) | 73 (62–79) | 0.358 |
| Administration of steroid (yes/no) | 15/25 | 36/34 | 0.159 |
| Etiology of ALI | |||
| Direct/indirect | 19/21 | 31/39 | 0.745 |
| Infection (sepsis) (yes/no) | 27/13 | 47/23 | 0.969 |
| Non-infection (non-sepsis) | |||
| Operation (yes/no) | 2/38 | 7/63 | 0.357 |
| Aspiration (yes/no) | 6/34 | 11/59 | 0.921 |
| Others (yes/no) | 5/35 | 5/65 | 0.347 |
| Number of failed organs | 2 (1–2) | 2 (1–2) | 0.124 |
| SOFA score | 10 (7–14) | 9 (6–10) | 0.212 |
| GOCA score | 6 (5–8) | 5 (4–7) | 0.219 |
| SIRS score | 3 (3–4) | 3 (2–3) | 0.063 |
| DIC score | 4 (2–5) | 3 (1–5) | 0.182 |
| PaO2/FIO2 ratio (mmHg) | 174.1 (130.1–233.1) | 142.9 (110.0–205.0) | 0.110 |
| WBC (×103/μL) | 12.75 (7.15–15.95) | 12.15 (7.29–17.04) | 0.857 |
| CRP (mg/dL) | 12.00 (5.64–20.42) | 16.29 (6.26–20.68) | 0.452 |
Note: Results are median values (interquartile ranges in parentheses).
Abbreviations: ALI, acute lung injury; SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; CRP, C-reactive protein.
The types of infections and causative pathogens in ALI patients with sepsis are shown in Table 3.
Table 3.
Types of infections and causative pathogens in acute lung injury patients with sepsis
| Parameter | N (%) |
|---|---|
| Infectious types | |
| Pneumonia | 33 (44.6) |
| Abdominal infection | 14 (18.9) |
| Urinary tract infection | 12 (16.2) |
| Bacterial pleuritis | 8 (10.8) |
| Others (neck abscess, gas gangrene, etc) | 7 (9.5) |
| Causative pathogens | |
| Pseudomonas aeruginosa | 15 (20.3) |
| Staphylococcus aureus | 13 (17.6) |
| Escherichia coli | 9 (12.2) |
| Enterococcus species | 9 (12.2) |
| Streptococcus pneumonia | 7 (9.5) |
| Klebsiella pneumonia | 5 (6.8) |
| Prevotella species | 4 (5.4) |
| Corynebacterium species | 4 (5.4) |
| Stenotrophomonas maltophilia | 3 (4.1) |
| Staphylococcus epidermidis | 2 (2.7) |
| Haemophilus influenzae | 1 (1.4) |
| Chryseobacterium meningosepticum | 1 (1.4) |
| Acinetobacter species | 1 (1.4) |
Sivelestat efficacy in ALI patients with SIRS
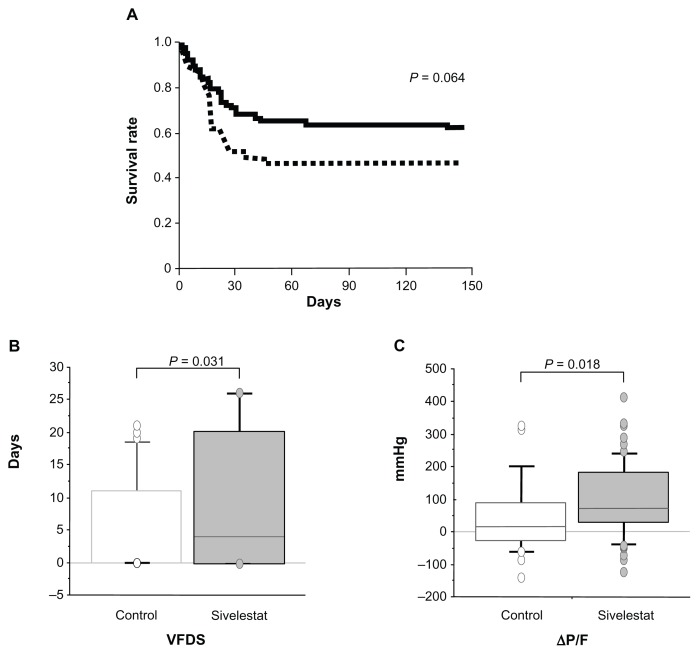
The survival rate of the sivelestat patients showed a tendency to be higher than that of the control patients, although the difference was not significant (P = 0.064) (Figure 1A). The VFDs and ΔP/F of the sivelestat patients were significantly higher than those of the control patients (P = 0.031 and 0.018, respectively) (Figure 1B and C).
Figure 1.
Clinical efficacy of sivelestat for acute lung injury patients with systemic inflammatory response syndrome. (A) Kaplan–Meier curves for acute lung injury patients with systemic inflammatory response syndrome who did or did not receive sivelestat. Statistical analysis was performed using the log-rank test. Solid line, sivelestat patients; dashed line, control patients. (B and C) Clinical efficacy of sivelestat based on ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after diagnosis of acute lung injury.
Note: Statistical analysis was performed using the Mann–Whitney U test.
Patient clinical factors favorable to sivelestat administration
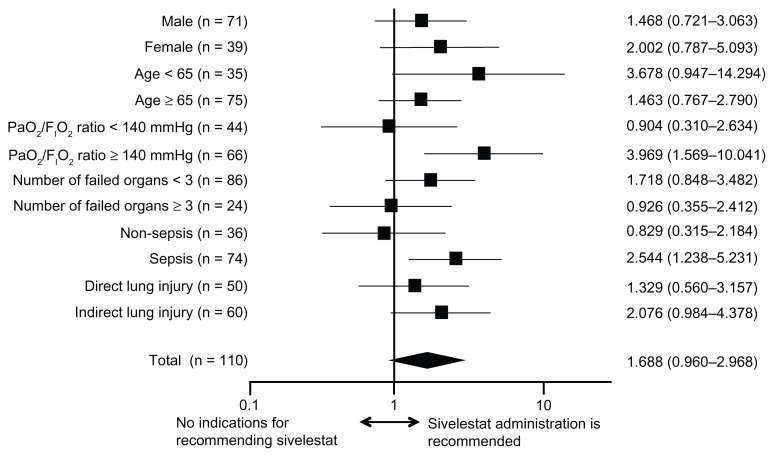
Sivelestat administration was significantly effective in ALI patients with a P/F ratio ≥ 140 mmHg and sepsis (P = 0.004 and 0.011, respectively) (Figure 2). Although statistical significance was not obtained, sivelestat administration was also associated with a trend toward prolonged survival of other subgroups, except for patients with P/F ratios < 140 mmHg, more than three failed organs, or without sepsis.
Figure 2.
Hazard ratios and 95% confidence intervals for survival based on the Cox proportional hazards model for assessing acute lung injury patients with systemic inflammatory response syndrome.
Efficacy of sivelestat in septic and non-septic ALI patients
The characteristics of septic and non-septic patients at the time of ALI diagnosis are shown in Table 4. There were 74 patients diagnosed with sepsis and 36 non-septic patients. Among the septic patients, the P/F of the control group was significantly higher than that of the sivelestat group. In non-septic patients, there were no significant differences for any of the variables of the treated and control patients.
Table 4.
Clinical characteristics of non-septic patients and septic patients
| Non-septic | Septic | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | Sivelestat | P | Control | Sivelestat | P | |
| Patients | 13 | 23 | 27 | 47 | ||
| Sex (male/female) | 7/6 | 16/7 | 0.346 | 16/11 | 32/15 | 0.444 |
| Age (yrs) | 73 (62–76) | 75 (64–81) | 0.365 | 70 (56–79) | 73 (61–77) | 0.570 |
| Etiology of ALI (direct/indirect) | 6/7 | 8/15 | 0.501 | 13/14 | 23/24 | 0.948 |
| Number of failed organs | 2 (1–3) | 2 (1–2) | 0.805 | 2 (1–3) | 1 (1–2) | 0.105 |
| Administration of steroid (yes/no) | 5/8 | 13/10 | 0.298 | 10/17 | 23/24 | 0.322 |
| SOFA score | 9 (5–13) | 8 (6–10) | 0.542 | 10 (7–14) | 9 (6–11) | 0.259 |
| GOCA score | 5 (4–7) | 6 (4–6) | 0.730 | 6 (5–8) | 5 (4–7) | 0.213 |
| SIRS score | 3 (3–4) | 3 (2–3) | 0.753 | 3 (3–4) | 3 (2–3) | 0.312 |
| DIC score | 5 (3–5) | 3 (2–4) | 0.096 | 4 (2–5) | 2 (1–5) | 0.556 |
| PaO2/FIO2 ratio (mmHg) | 168.3 (115.9–178.8) | 135.2 (103.7–222.2) | 0.693 | 195.7 (138.5–250.5) | 144.0 (120.8–197.8) | 0.046 |
| WBC (×103/μL) | 12.70 (3.30–14.60) | 11.44 (9.06–14.41) | 0.521 | 12.80 (10.08–19.15) | 12.20 (6.68–17.68) | 0.419 |
| CRP (mg/dL) | 6.88 (3.28–16.01) | 17.48 (4.81–20.29) | 0.134 | 13.59 (7.16–24.98) | 15.29 (6.91–21.62) | 0.978 |
Note: Results are median values (interquartile ranges in parentheses).
Abbreviations: ALI, acute lung injury; SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; CRP, C-reactive protein.
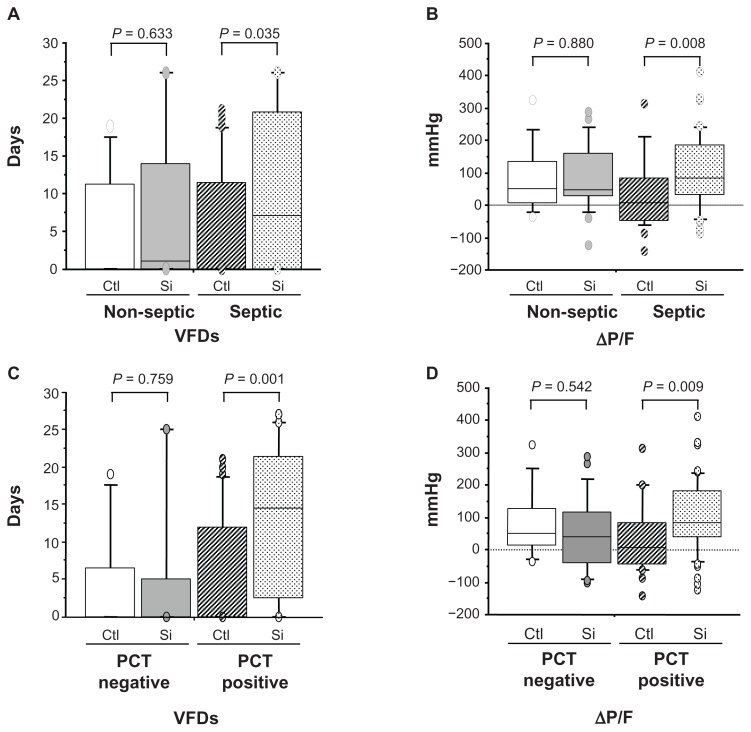
The number of VFDs and ΔP/F of the septic patients receiving sivelestat were significantly higher than those of the septic control patients (P = 0.035 and 0.008, respectively) (Figure 3A and B). There were no significant differences in the number of VFDs and ΔP/F of the non-septic control and sivelestat patients (Figure 3A and B).
Figure 3.
Clinical efficacy of sivelestat based on ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after sivelestat administration for acute lung injury patients with systemic inflammatory response syndrome who were non-septic, septic, negative for procalcitonin (PCT), and positive for PCT. (A and C) VFD for patients who were non-septic, septic, negative for PCT, and positive for PCT. (B and D) ΔP/F for patients who were non-septic, septic, negative for PCT, and positive for PCT.
Notes: PCT levels higher than 0.5 ng/mL were considered PCT positive. Statistical analysis was performed using the Mann–Whitney U test.
Abbreviations: Ctl, control patients; Si, sivelestat patients.
Analysis of predictive parameters of mortality in patients with or without sepsis
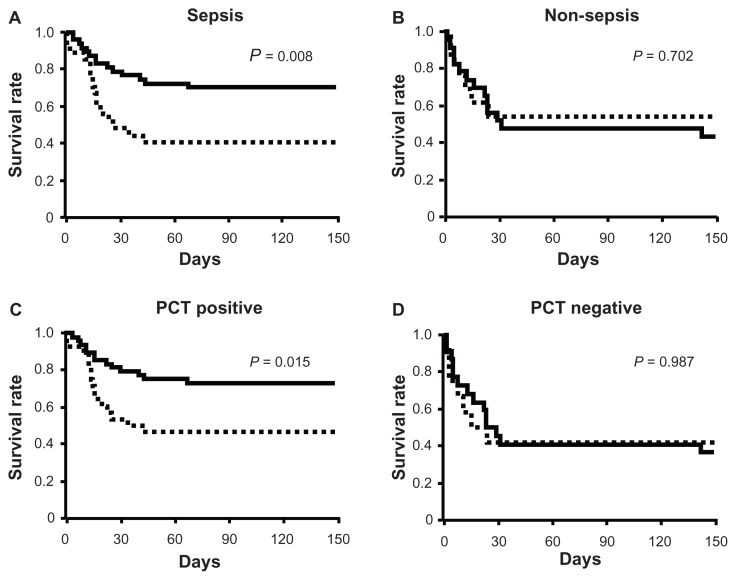
Univariate analysis showed that the number of failed organs; sivelestat administration; and the SOFA, GOCA, and DIC scores at diagnosis were significantly correlated with mortality in septic patients (all P < 0.05) (Table 5). Multivariate analysis demonstrated that sivelestat administration and the GOCA score were predictors of mortality in septic patients (all P < 0.05) (Table 5). Kaplan–Meier curves revealed significantly prolonged survival in the sivelestat group compared with the control group in septic patients (P = 0.008) (Figure 4A). In non-septic patients, the SOFA and GOCA scores at diagnosis, but not sivelestat administration, were significantly correlated with mortality (Table 6). In addition, there were no significant differences in survival rate between the control and sivelestat groups (Figure 4B).
Table 5.
The hazard ratios and 95% confidence intervals (CIs) for mortality based on univariate and multivariate Cox analysis in septic acute lung injury patients
| Parameter | Hazard ratio | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Univariate | ||||
| Male | 1.260 | 0.607 | 2.617 | 0.535 |
| Age | 1.023 | 0.994 | 1.052 | 0.124 |
| Indirect lung injury | 1.364 | 0.662 | 2.809 | 0.400 |
| Number of failed organs | 2.704 | 1.699 | 4.305 | <0.001 |
| Administration of sivelestat | 0.393 | 0.191 | 0.808 | 0.011 |
| Administration of steroid | 0.909 | 0.441 | 1.871 | 0.795 |
| SOFA score | 1.185 | 1.106 | 1.268 | <0.001 |
| GOCA score | 1.553 | 1.303 | 1.851 | <0.001 |
| SIRS score | 1.321 | 0.785 | 2.224 | 0.294 |
| DIC score | 1.317 | 1.136 | 1.526 | 0.003 |
| PaO2/FIO2 ratio | 1.004 | 0.998 | 1.011 | 0.145 |
| WBC | 1.000 | 1.000 | 1.000 | 0.550 |
| CRP | 0.969 | 0.936 | 1.005 | 0.087 |
| Multivariate | ||||
| Number of organ failures | 1.185 | 0.604 | 2.323 | 0.621 |
| Administration of sivelestat | 0.366 | 0.171 | 0.783 | 0.010 |
| SOFA score | 1.000 | 0.876 | 1.143 | 0.995 |
| GOCA score | 1.448 | 1.042 | 2.012 | 0.028 |
| DIC score | 1.106 | 0.929 | 1.317 | 0.257 |
Abbreviations: SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; CRP, C-reactive protein.
Figure 4.
Kaplan–Meier curves for acute lung injury patients with systemic inflammatory response syndrome who were septic (A), non-septic (B), positive for procalcitonin (PCT) (C), and negative for PCT (D).
Notes: PCT levels higher than 0.5 ng/mL were considered PCT positive. Statistical analysis was performed using the log-rank test. Solid line, sivelestat patients; dashed line, control patients.
Table 6.
The hazard ratios and 95% confidence intervals for mortality based on univariate and multivariate Cox analysis in non-septic acute lung injury patients
| Parameter | Hazard ratio | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Univariate | ||||
| Male | 0.824 | 0.313 | 2.170 | 0.695 |
| Age | 1.023 | 0.984 | 1.064 | 0.250 |
| Indirect lung injury | 0.905 | 0.364 | 2.252 | 0.831 |
| Number of failed organs | 1.058 | 0.555 | 2.015 | 0.864 |
| Administration of sivelestat | 1.206 | 0.458 | 3.175 | 0.705 |
| Administration of steroid | 2.136 | 0.840 | 5.433 | 0.111 |
| SOFA score | 1.137 | 1.003 | 1.288 | 0.044 |
| GOCA score | 1.429 | 1.105 | 1.848 | 0.007 |
| SIRS score | 0.902 | 0.494 | 1.647 | 0.738 |
| DIC score | 1.184 | 0.965 | 1.453 | 0.106 |
| PaO2/FIO2 ratio | 0.991 | 0.982 | 1.001 | 0.070 |
| WBC | 1.000 | 1.000 | 1.000 | 0.391 |
| CRP | 0.986 | 0.935 | 1.041 | 0.618 |
| Multivariate | ||||
| SOFA score | 0.958 | 0.787 | 1.167 | 0.670 |
| GOCA score | 1.530 | 1.023 | 2.288 | 0.038 |
Abbreviations: ALI, acute lung injury; SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; CRP, C-reactive protein.
Efficacy of sivelestat in PCT-positive and PCT-negative patients
Patients were stratified according to whether they were PCT positive or negative, and the efficacy of sivelestat was evaluated for each group. Patient characteristics are shown in Table 7. In both PCT-positive and -negative patients, there were no significant differences in any clinical variable between the control and the sivelestat groups.
Table 7.
Clinical characteristics of procalcitonin (PCT)-negative patients and PCT-positive patients
| PCT negative | PCT positive | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | Sivelestat | P | Control | Sivelestat | P | |
| Patients | 12 | 22 | 28 | 48 | ||
| Sex (male/female) | 5/7 | 16/6 | 0.075 | 18/10 | 32/16 | 0.833 |
| Age (yrs) | 71 (62–76) | 74 (69–81) | 0.387 | 70 (57–79) | 73 (61–77) | 0.576 |
| Etiology of ALI (direct/indirect) | 5/7 | 9/13 | 0.966 | 14/14 | 22/26 | 0.726 |
| Number of failed organs | 2 (1–3) | 2 (1–2) | 0.843 | 2 (1–3) | 2 (1–2) | 0.096 |
| Administration of steroid (yes/no) | 4/8 | 14/8 | 0.091 | 11/17 | 22/26 | 0.578 |
| SOFA score | 10 ( 5–14) | 8 (6–10) | 0.601 | 10 (7–13) | 9 (6–10) | 0.232 |
| GOCA score | 6 (4–8) | 6 (4–8) | 0.732 | 6 (5–8) | 5 (4–7) | 0.202 |
| SIRS score | 4 (3–4) | 3 (2–3) | 0.056 | 3 (3–4) | 3 (2–3) | 0.369 |
| DIC score | 4 (3–5) | 3 (2–5) | 0.221 | 4 (2–5) | 2 (1–5) | 0.383 |
| PaO2/FIO2 ratio (mmHg) | 170.8 (107.8–187.5) | 130.4 (102.6–200.0) | 0.829 | 184.6 (141.1–245) | 150.1 (127.0–205.5) | 0.090 |
| WBC (×103/μL) | 9.15 (3.20–14.90) | 11.92 (9.00–14.50) | 0.428 | 13.30 (10.25–17.25) | 12.15 (6.75–17.60) | 0.383 |
| CRP (mg/dL) | 5.94 (2.56–11.82) | 16.88 (4.32–18.74) | 0.066 | 14.6 (9.23–24.96) | 15.22 (6.50–22.78) | 0.667 |
Note: Results are median values (interquartile ranges in parentheses).
Abbreviations: ALI, acute lung injury; SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; PLT, platelet; CRP, C-reactive protein.
Among PCT-positive patients, the number of VFDs and ΔP/F of the sivelestat group were significantly higher than those of the control group (P = 0.001 and 0.009, respectively) (Figure 3C and D). Among PCT-negative patients, there were no significant differences in these values between the control and sivelestat patients (Figure 3C and D).
Analysis of predictive parameters of mortality in PCT-positive and PCT-negative patients
Univariate analysis showed that the number of failed organs; sivelestat administration; and the SOFA, GOCA, and DIC scores at diagnosis were significantly correlated with mortality in PCT-positive patients (all P < 0.05) (Table 8). Multivariate analysis showed that sivelestat administration was a predictor of mortality only in PCT-positive patients (P = 0.030) (Table 8). Kaplan–Meier curves showed that sivelestat administration significantly prolonged survival in comparison to the control group in PCT-positive patients (P = 0.015) (Figure 4C). Although the data are not shown, the SOFA (HR for death, 1.176; 95% CI, 1.057–1.308; P = 0.003), GOCA (HR for death, 1.472; 95% CI, 1.185–1.830; P < 0.001), and DIC scores (HR for death, 1.344; 95% CI, 1.089–1.659; P = 0.006) at diagnosis, but not sivelestat administration (HR for survival, 0.992; 95% CI, 0.400–2.462; P = 0.987), were significantly correlated with survival in PCT-negative patients. In addition, there were no significant differences in the survival rate between the control and the sivelestat groups (Figure 4D).
Table 8.
The hazard ratios and 95% confidence intervals (CIs) for mortality based on univariate and multivariate Cox analysis in procalcitonin-positive patients
| Parameter | Hazard ratio | 95% CI | P | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Univariate | ||||
| Male | 1.246 | 0.583 | 2.682 | 0.570 |
| Age | 1.030 | 0.999 | 1.062 | 0.056 |
| Indirect lung injury | 1.289 | 0.610 | 2.725 | 0.506 |
| Number of organ failure | 2.616 | 1.616 | 4.234 | <0.001 |
| Administration of sivelestat | 0.409 | 0.194 | 0.862 | 0.019 |
| Administration of steroid | 0.942 | 0.446 | 1.992 | 0.876 |
| SOFA score | 1.173 | 1.091 | 1.261 | <0.001 |
| GOCA score | 1.511 | 1.253 | 1.822 | <0.001 |
| SIRS score | 1.451 | 0.841 | 2.504 | 0.181 |
| DIC score | 1.269 | 1.092 | 1.475 | 0.002 |
| PaO2/FIO2 ratio | 1.003 | 0.997 | 1.010 | 0.323 |
| WBC | 1.000 | 1.000 | 1.000 | 0.582 |
| CRP | 0.981 | 0.947 | 1.017 | 0.295 |
| Multivariate | ||||
| Number of organ failure | 1.275 | 0.635 | 2.561 | 0.494 |
| Administration of sivelestat | 0.422 | 0.194 | 0.919 | 0.030 |
| SOFA score | 1.001 | 0.868 | 1.154 | 0.992 |
| GOCA score | 1.365 | 0.959 | 1.941 | 0.084 |
| DIC score | 1.096 | 0.917 | 1.310 | 0.315 |
Abbreviations: ALI, acute lung injury; SOFA score, sequential organ failure assessment score; GOCA score, gas exchange, organ failure, cause, associated disease score; SIRS, systemic inflammatory response syndrome; DIC, disseminated intravascular coagulation; WBC, white blood cell; CRP, C-reactive protein.
Discussion
Our study showed that treatment with sivelestat improved the respiratory status of ALI patients with SIRS. When survival was evaluated as a function of sivelestat efficacy, drug administration was significantly more effective for patients with better respiratory function at diagnosis or with sepsis than for patients without these features. In addition, sivelestat significantly prolonged survival, led to a greater number of VFDs, and increased ΔP/F in septic patients and PCT-positive patients. We propose that sivelestat treatment may improve survival for ALI patients with sepsis, and that the initial serum PCT level can be used to indicate whether to use sivelestat.
Sivelestat improved the respiratory status of patients with ALI associated with SIRS in our study. Others have also reported that sivelestat improved the lung injury score, reduced the duration of mechanical ventilation, and shortened the time in the ICU of ALI patients with SIRS.13 In addition, Aikawa et al reported that sivelestat for ALI patients with SIRS contributed to early weaning from mechanical ventilation.1 We have also shown that the number of VFDs and ΔP/F were significantly higher in the sivelestat group than in the control group. The survival rate tended to be higher in patients treated with sivelestat group than in the control patients, although statistical significance was not achieved.
The results of STRIVE, a prospective, randomized, double-blind, placebo-controlled, multicenter trial, indicated that sivelestat probably did not have beneficial effects on the respiratory function and survival of ALI/ARDS patients, whereas Phase III and IV Japanese studies showed that sivelestat was beneficial.1,13,14 This discrepancy might be due to differences in the characteristics of study patients such as age, baseline respiratory status, the number of non-pulmonary failed organs, and sepsis.1,14,26 Zeiher et al reported that the patients enrolled in the Japanese Phase III study had a smaller age distribution and had less severe respiratory problems than those in STRIVE.14 In addition, patients with organ failure involving four or more organs were excluded from the Japanese Phase III study.13 It was reported that a post hoc analysis of the STRIVE patient subgroup that met the Phase III inclusion and exclusion criteria and had a mean lung injury score ≤ 2.5, revealed favorable trends in mortality and VFDs for the patients receiving sivelestat.14 In contrast, Hayakawa et al suggested that differences in the numbers of septic patients may have led to the discordant results among the studies.26 There were significant differences in the proportion of septic patients in each study: STRIVE (58%) and in the Japanese Phase III (69%) and Phase IV (71%) studies (all P < 0.01). In our study, sivelestat was more effective in patients with P/F ≥ 140 mmHg or sepsis but less effective in patients with P/F < 140 mmHg, more than three failed organs, or without sepsis. Based on our results and previous reports, the baseline respiratory function, number of failed organs, and septic status may affect the efficacy of sivelestat.
Sivelestat may be beneficial for ALI patients with sepsis. It was reported that sivelestat shortened time spent in the ICU and might be an independent predictor of survival in septic patients with ALI and DIC.26 Tsuboko et al also reported that sivelestat reduced the duration of artificial ventilation and improved pulmonary function and the multiple organ dysfunction score in patients with ALI/ARDS following surgery for abdominal sepsis.16 Our study revealed that sivelestat prolonged survival in septic ALI patients and increased the number of VFDs and ΔP/F. Another important finding of our study was that sivelestat did not improve the respiratory function or the outcome of non-septic patients. Therefore, the reasons for the lack of efficacy of sivelestat in non-septic patients need to be elucidated.
Sivelestat may be beneficial for PCT-positive patients with ALI. Based on the results of our study, we believe that sepsis may be closely associated with survival in ALI patients with SIRS. Differentiating sepsis from noninfectious SIRS is difficult, because sepsis is a complex, heterogeneous disorder.27 Previous reports have shown that PCT was a useful diagnostic marker for sepsis, and that PCT levels are closely correlated with the severity of sepsis.27–30 Therefore, we evaluated whether the initial serum PCT level was a useful indicator for the administration of sivelestat. In PCT-positive patients, the survival rate, number of VFDs, and ΔP/F of the sivelestat group were significantly higher than in the control group (P = 0.015, 0.001, and 0.009, respectively), whereas sivelestat efficacy was not demonstrated for PCT-negative patients. In addition, multivariate analysis showed that sivelestat administration was a predictor of mortality only in PCT-positive patients. These results suggest that the initial serum PCT level may be an indicator for whether to administer sivelestat to ALI patients with SIRS.
The relationship between PCT and sivelestat remains unclear. It was reported that interleukin (IL)-1β and tumor necrosis factor-α, which have been ascribed significant roles in the cytokine mediation of sepsis and septic shock, acted as potent stimulators of calcitonin messenger RNA expression and procalcitonin synthesis.31 Further, Suda et al reported that treatment with sivelestat suppressed the serum concentrations of IL-1β and tumor necrosis factor-α in septic animals.32 However, further studies are needed to clarify the relationship between procalcitonin and the neutrophil elastase inhibitor.
Sivelestat may be useful for patients with ALI and sepsis, as it has been reported that sivelestat might attenuate the vicious inflammatory cycle; reduce the sequestration, infiltration and activation of inflammatory cells; and suppress IL-8 and high-mobility group box chromosomal protein 1 (HMGB1) protein expression.32 Activated alveolar macrophages in sepsis produce various mediators such as IL-8 and HMGB1 that attract neutrophils to the lungs.33–37 Neutrophil elastase released from activated neutrophils stimulates protease-activated receptor 2 and induces production of proinflammatory cytokines such as IL-8, which leads to amplified sequestration, activation of neutrophils, and exacerbation of inflammation.33 Suda et al demonstrated that sivelestat significantly decreased the number of HMGB1- and IL-8-positive cells in the lungs of rats with sepsis.32 It was also reported that sivelestat administration decreased IL-8 serum levels in septic ALI patients.15
Limitations
Our study was a retrospective analysis, and the number of patients was small. There were no restrictions on the use of other drugs in addition to sivelestat, and we did not evaluate other therapies except for the use of steroids. We did not evaluate the effect of steroid administration on P/F or other parameters. Although there was no significant difference in the number of patients receiving steroid therapy in the control and sivelestat groups, additional studies examining the effects of steroid and sivelestat coadministration to ALI patients are needed. We did not evaluate any other inflammatory mediators. Finally, the PCT measurements were performed using semi-quantitative instead of quantitative analysis.
Conclusion
Our study demonstrated that sivelestat might improve the ALI and survival of septic patients. The initial serum PCT level may be useful for indicating whether to use sivelestat for ALI patients with SIRS. However, a large prospective study is needed to clarify the usefulness of sivelestat for ALI patients with sepsis.
Footnotes
Disclosure
The work reported here was undertaken at: Department of Integrated Medicine and Informatics, Ehime University Graduate School of Medicine; Department of Respiratory Medicine, Sumitomo Besshi Hospital; Department of Respiratory Medicine, Ehime Prefectural Central Hospital; Department of Respiratory Medicine, Matsuyama Red Cross Hospital, Intensive Care Division, Ehime University Hospital; and Department of Emergency Medicine, School of Medicine, Ehime University.
The authors declare no conflicts of interest in this work.
References
- 1.Aikawa N, Ishizaka A, Hirasawa H, et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm Pharmacol Ther. 2011;24(5):549–554. doi: 10.1016/j.pupt.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 3.Janoff A, White R, Carp H, Harel S, Dearing R, Lee D. Lung injury induced by leukocytic proteases. Am J Pathol. 1979;97(1):111–136. [PMC free article] [PubMed] [Google Scholar]
- 4.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- 5.Idell S, Kucich U, Fein A, et al. Neutrophil elastase-releasing factors in bronchoalveolar lavage from patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132(5):1098–1105. doi: 10.1164/arrd.1985.132.5.1098. [DOI] [PubMed] [Google Scholar]
- 6.Donnelly SC, MacGregor I, Zamani A, et al. Plasma elastase levels and the development of the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(5):1428–1433. doi: 10.1164/ajrccm.151.5.7735596. [DOI] [PubMed] [Google Scholar]
- 7.Petty TL. Protease mechanisms in the pathogenesis of acute lung injury. Ann N Y Acad Sci. 1991;624:267–277. doi: 10.1111/j.1749-6632.1991.tb17025.x. [DOI] [PubMed] [Google Scholar]
- 8.Ueno H, Hirasawa H, Oda S, Shiga H, Nakanishi K, Matsuda K. Coagulation/fibrinolysis abnormality and vascular endothelial damage in the pathogenesis of thrombocytopenic multiple organ failure. Crit Care Med. 2002;30(10):2242–2248. doi: 10.1097/00003246-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 9.McGill SN, Ahmed NA, Christou NV. Endothelial cells: role in infection and inflammation. World J Surg. 1998;22(2):171–178. doi: 10.1007/s002689900366. [DOI] [PubMed] [Google Scholar]
- 10.Russell JA. Management of sepsis. N Engl J Med. 2006;355(16):1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 11.Kawabata K, Suzuki M, Sugitani M, Imaki K, Toda M, Miyamoto T. ONO-5046, a novel inhibitor of human neutrophil elastase. Biochem Biophys Res Commun. 1991;177(2):814–820. doi: 10.1016/0006-291x(91)91862-7. [DOI] [PubMed] [Google Scholar]
- 12.Iba T, Kidokoro A, Fukunaga M, Takuhiro K, Yoshikawa S, Sugimotoa K. Pretreatment of sivelestat sodium hydrate improves the lung microcirculation and alveolar damage in lipopolysaccharide-induced acute lung inflammation in hamsters. Shock. 2006;26(1):95–98. doi: 10.1097/01.shk.0000223126.34017.d9. [DOI] [PubMed] [Google Scholar]
- 13.Tamakuma S, Ogawa M, Aikawa N, et al. Relationship between neutrophil elastase and acute lung injury in humans. Pulm Pharmacol Ther. 2004;17(5):271–279. doi: 10.1016/j.pupt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Zeiher BG, Artigas A, Vincent JL, et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32(8):1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 15.Endo S, Sato N, Yaegashi Y, et al. Sivelestat sodium hydrate improves septic acute lung injury by reducing alveolar dysfunction. Res Commun Mol Pathol Pharmacol. 2006;119(1–6):53–65. [PubMed] [Google Scholar]
- 16.Tsuboko Y, Takeda S, Mii S, et al. Clinical evaluation of sivelestat for acute lung injury/acute respiratory distress syndrome following surgery for abdominal sepsis. Drug Des Devel Ther. 2012;6:273–278. doi: 10.2147/DDDT.S36436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 18.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 19.Gando S, Iba T, Eguchi Y, et al. Japanese Association for Acute Medicine Disseminated Intravascular Coagulation (JAAM DIC) Study Group. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34(3):625–631. doi: 10.1097/01.ccm.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 20.Singh RK, Baronia AK, Sahoo JN, et al. Prospective comparison of new Japanese Association for Acute Medicine (JAAM) DIC and International Society of Thrombosis and Hemostasis (ISTH) DIC score in critically ill septic patients. Thromb Res. 2012;129(4):e119–e125. doi: 10.1016/j.thromres.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 21.Levy MM, Fink MP, Marshall JC, et al. SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 23.Jegal Y, Lee SI, Lee KH, et al. The clinical efficacy of GOCA scoring system in patients with acute respiratory distress syndrome. J Korean Med Sci. 2008;23(3):383–389. doi: 10.3346/jkms.2008.23.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Delèvaux I, André M, Colombier M, et al. Can procalcitonin measurement help in differentiating between bacterial infection and other kinds of inflammatory processes? Ann Rheum Dis. 2003;62(4):337–340. doi: 10.1136/ard.62.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayakawa M, Katabami K, Wada T, et al. Sivelestat (selective neutrophil elastase inhibitor) improves the mortality rate of sepsis associated with both acute respiratory distress syndrome and disseminated intravascular coagulation patients. Shock. 2010;33(1):14–18. doi: 10.1097/SHK.0b013e3181aa95c4. [DOI] [PubMed] [Google Scholar]
- 27.Tsalik EL, Jaggers LB, Glickman SW, et al. Discriminative value of inflammatory biomarkers for suspected sepsis. J Emerg Med. 2011;43(1):97–106. doi: 10.1016/j.jemermed.2011.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitaka C. Clinical laboratory differentiation of infectious versus non-infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351(1–2):17–29. doi: 10.1016/j.cccn.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 29.Luzzani A, Polati E, Dorizzi R, Rungatscher A, Pavan R, Merlini A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit Care Med. 2003;31(6):1737–1741. doi: 10.1097/01.CCM.0000063440.19188.ED. [DOI] [PubMed] [Google Scholar]
- 30.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34(7):1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 31.Linscheid P, Seboek D, Nylen ES, et al. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology. 2003;144(12):5578–5584. doi: 10.1210/en.2003-0854. [DOI] [PubMed] [Google Scholar]
- 32.Suda K, Takeuchi H, Hagiwara T, et al. Neutrophil elastase inhibitor improves survival of rats with clinically relevant sepsis. Shock. 2010;33(5):526–531. doi: 10.1097/SHK.0b013e3181cc064b. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, Yang H, Sakuragi T, et al. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L583–L590. doi: 10.1152/ajplung.00091.2005. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 35.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165(6):2950–2954. doi: 10.4049/jimmunol.165.6.2950. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255(3):320–331. doi: 10.1111/j.1365-2796.2003.01302.x. [DOI] [PubMed] [Google Scholar]
- 37.Ueno H, Matsuda T, Hashimoto S, et al. Contributions of high mobility group box protein in experimental and clinical acute lung injury. Am J Respir Crit Care Med. 2004;170(12):1310–1316. doi: 10.1164/rccm.200402-188OC. [DOI] [PubMed] [Google Scholar]