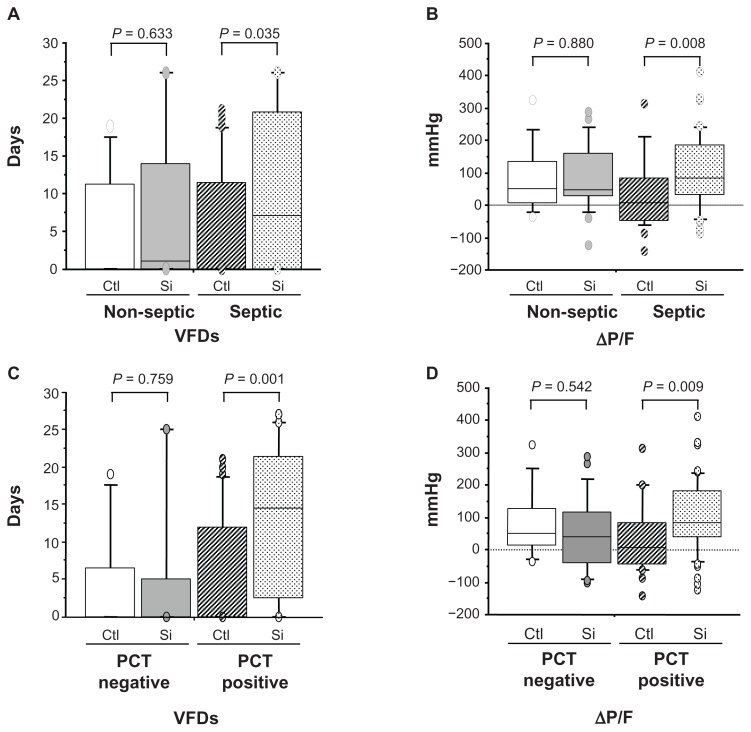
Figure 3.
Clinical efficacy of sivelestat based on ventilator-free days (VFDs) and changes in PaO2/FIO2 (ΔP/F) before and 7 days after sivelestat administration for acute lung injury patients with systemic inflammatory response syndrome who were non-septic, septic, negative for procalcitonin (PCT), and positive for PCT. (A and C) VFD for patients who were non-septic, septic, negative for PCT, and positive for PCT. (B and D) ΔP/F for patients who were non-septic, septic, negative for PCT, and positive for PCT.
Notes: PCT levels higher than 0.5 ng/mL were considered PCT positive. Statistical analysis was performed using the Mann–Whitney U test.
Abbreviations: Ctl, control patients; Si, sivelestat patients.