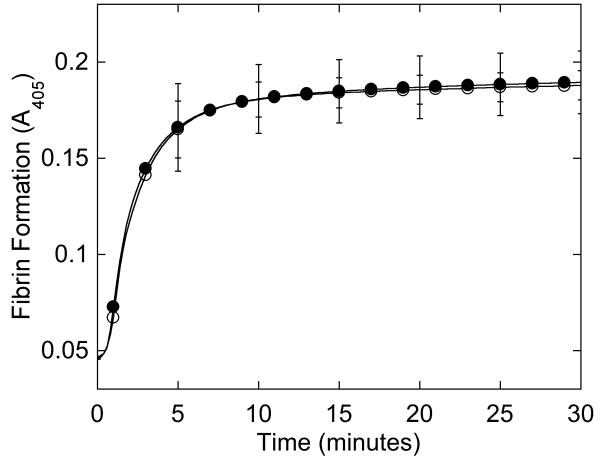
Figure 2. Purified fibrinogen contains all three polypeptide chains at the expected molecular weights and has normal clotting function.
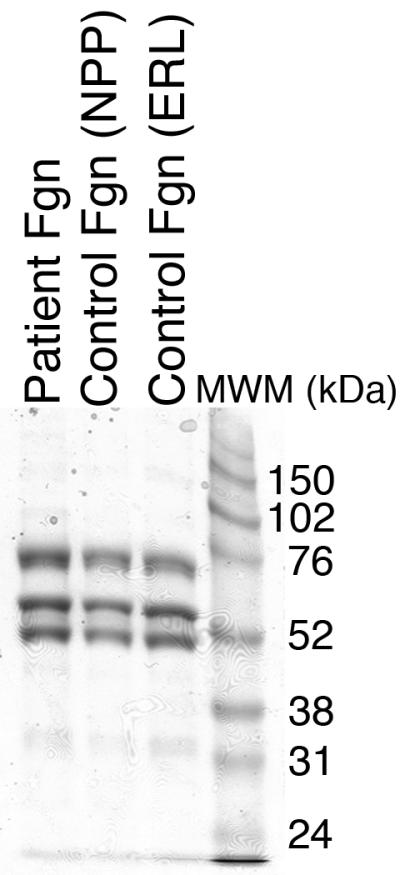
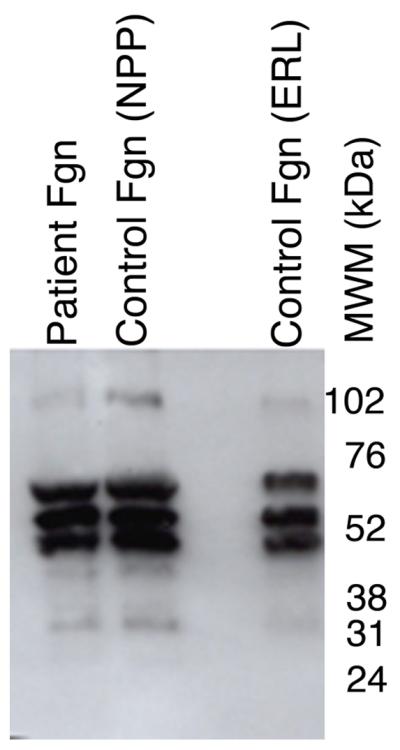
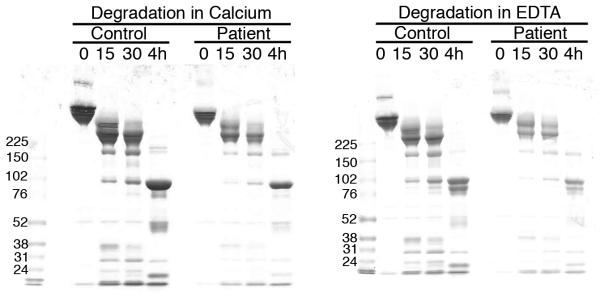
A-B) Fibrinogen was precipitated from plasma, and equivalent amounts (10 μg) were separated by 10% SDS-PAGE under reducing conditions. A) Coomassie Brilliant Blue R250-stained gel. B) Western blot using rabbit anti-human fibrinogen polyclonal antibody. Lanes are: (P) Patient fibrinogen, (N) Fibrinogen precipitated from NPP in-house, and (C) Commercial fibrinogen. Molecular weight markers are indicated. C) Purified fibrinogen (0.5 mg/mL in HBS) was pre-incubated with CaCl2 (5 mM, final) for 1 minute, and clotted by adding thrombin (5 nM, final). Panel C shows mean and standard deviation from three separate reactions performed in triplicate at room temperature and followed by turbidity at 405 nm. Symbols are: NPP (○) and patient plasma (●); note that NPP and patient fibrinogen polymerization curves are identical. (D) Purified fibrinogen was incubated with plasmin in the presence of 1 mM CaCl2 or 5 mM EDTA (final, as indicated) at 37°C. Timed aliquots were quenched with SDS-PAGE loading dye, boiled, separated by non-reducing 4-12% SDS-PAGE, and stained by Coomassie Brilliant Blue R250.