Abstract
Sleep of good quantity and quality is considered a biologically important resource necessary to maintain homeostasis of pain-regulatory processes. To assess the role of chronic sleep disturbances in pain processing, we conducted laboratory pain testing in subjects with primary insomnia.
Seventeen participants with primary insomnia (mean±SEM 22.6±0.9 years, 11 women) were individually matched with 17 healthy participants. All participants completed daily sleep and pain diaries over a 2-week period. Laboratory pain testing was conducted in a controlled environment and included (1) warmth detection threshold testing, (2) pain sensitivity testing (threshold detection for heat and pressure pain), and (3) tests to access pain-modulatory mechanisms (temporal summation and pain inhibition).
Primary insomnia subjects reported experiencing spontaneous pain on twice as many days as healthy controls during the at-home recording phase (p<0.05). During laboratory testing, primary insomnia subjects had lower pain thresholds than healthy controls (p<0.05 for heat pain detection threshold, p<0.08 for pressure pain detection threshold). Unexpectedly, pain facilitation, as assessed with temporal summation of pain responses, was reduced in primary insomnia compared to healthy controls (p<0.05). Pain inhibition, as assessed with the diffuse noxious inhibitory control paradigm (DNIC), was attenuated in insomnia subjects when compared to controls (p<0.05). Based on these findings, we hypothesize that pain-inhibitory circuits in patients with insomnia are in a state of constant activation to compensate for ongoing subclinical pain. This constant activation ultimately results in a ceiling effect of pain-inhibitory efforts, as indicated by the inability of the system to adequately function during challenge.
Keywords: Insomnia, sleep, pain, temporal summation, diffuse noxious inhibitory control (DNIC)
INTRODUCTION
Good sleep quantity and quality appear to have a physiologically important role in the regulation of pain processing (for a review, see (Haack et al., 2009). Substantial clinical evidence provides support for a bi-directional relationship between sleep quantity/quality and spontaneous pain (Affleck et al., 1996; Lee et al., 2009). Experimental studies in healthy volunteers demonstrate that sleep restriction for one or more days, or total sleep deprivation, leads to the development of a new onset spontaneous pain (Haack and Mullington, 2005; Haack et al., 2007). Additional experimental human studies have shown that restriction of sleep at various stages for one or more nights, or total sleep deprivation, increases sensitivity to experimentally evoked pain (Cooperman et al., 1934; Moldofsky and Scarisbrick, 1976; Older et al., 1998; Onen et al., 2001; Kundermann et al., 2004; Roehrs et al., 2006; Smith et al., 2007).
In order to better understand the role of sleep in pain processing, it is important to assess whether sleep affects the ability to centrally modulate pain. Deficiencies in the capacity to modulate pain appear to contribute to the susceptibility to acquire a pain disorder (Pud et al., 2009; van Wijk and Veldhuijzen, 2010). Central pain modulation originates from a network of descending pathways projecting from various cerebral areas to the dorsal horn, where the transfer of nociceptive input is then either facilitated or inhibited (Millan, 2002). Methods that have previously been used to address questions about pain modulatory processes are the diffuse noxious inhibitory control (DNIC) paradigm (Le Bars et al., 1979), in which the descending inhibitory responses are challenged during a conditioning pain-inducing stimulus, and the paradigm of temporal summation of pain, which is utilized to assess a potential mechanism of central pain facilitation (Herrero et al., 2000). Abnormalities in pain modulatory processes have been reported in a variety of pain syndromes, include arthritis, chronic tension-type headache, migraine, temporomandibular disorder (TMD), irritable bowel syndrome, neuropathic pain, and fibromyalgia (Milanov and Bogdanova, 2003; van Wijk and Veldhuijzen, 2010).
Though pain-modulatory mechanisms have rarely been studied in the context of insufficient sleep, there is some recent clinical evidence in TMD patients that altered pain-modulatory systems, as seen by a lower ability to inhibit pain, are associated with poor sleep, specifically, lower sleep efficiency and shorter total sleep time (Edwards et al., 2009). Further, in an experimental setting, Smith and colleagues showed that healthy women have decreased pain-inhibitory capacity after three nights of experimentally fragmented sleep (Smith et al., 2007). While these studies provide support for the relationship between sleep and pain at multiple levels (e.g., spontaneous pain, pain sensitivity, pain modulatory mechanisms), they are based on the study of either healthy participants or clinical pain populations, and therefore cannot address how alterations in sleep quality/quantity may lead to changes in pain processing independent from co-morbid illness and other factors. The current study tested the role of chronic sleep disturbances on alterations in pain processing by conducting experimental pain testing in otherwise healthy subjects with primary insomnia and a healthy comparison sample.
METHODS
Participants
Seventeen participants with primary insomnia and 17 individually age- and sex- matched healthy controls with good quantity/quality sleep completed the study (see Table 1). Participants were recruited through advertisements on public transportation and radio, as well as through online advertisements and paper fliers posted at Boston area colleges. Participants with insomnia met full diagnostic criteria for primary insomnia disorder based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR, (American Psychiatric Association, 2000). Insomnia participants were required to have difficulties initiating sleep, maintaining sleep, or waking up too early for at least one month in duration that was not caused by a medical or psychiatric conditions, or substance use. In addition, the participant’s sleep disturbances were required to result in substantial distress or daytime impairment in all domains of functioning. The Structured Interview for Sleep Disorders was used to assist in diagnostic assessment of primary insomnia (Schramm et al., 1993).
Table 1.
Study characteristics.
| Measure/Unit | Insomnia | Control | p-value | |
|---|---|---|---|---|
| N | 17 | 17 | 1.00 | |
| Sex | Female/Male | 11/6 | 11/6 | 1.00 |
| Race | White/Black/Asian/Other | 16/0/1/0 | 15/1/1/0 | 0.39 |
| Ethnicity | Non-Hispanic/Hispanic | 16/1 | 14/3 | 0.60 |
| Age (yrs) | Mean±SEM | 22.7±0.9 | 24.4±0.9 | 0.17 |
| Range | 18–31 | 20–32 | ||
| Screening BMI (kg/m2) | Mean±SEM | 23.3±0.7 | 23.1±0.9 | 0.90 |
| Range | 18.3–28.4 | 18.3–33.7 |
Additional questionnaires were used to assess sleep-wake habits and medical history (SF-36, Ware et al., 2000), Pittsburgh Sleep Quality Index (PSQI, Buysse et al., 1989), Patient Health Questionnaire (PHQ-I, Kroenke et al., 2001), and the research version of the Structured Clinical Interview for DSM-IV-TR Axis I disorders (SCID, First et al., 2004). Exclusion criteria were the presence of any Axis I disorder (e.g., major depressive disorder, generalized anxiety disorder, alcohol abuse) in the last six months, or any medical disorders, ongoing painful conditions, substance or sleep disorders other than primary insomnia. All participants were also required to provide a statement from their primary care physician indicating that the participants were healthy except for primary insomnia.
Study protocol
The institutional review board at the Beth Israel Deaconess Medical Center approved the study and participants provided written informed consent for study participation. Following an initial screening visit (Visit 1), eligible participants were provided with an actigraph and a sleep diary to record their habitual sleep-wake habits across a 2-week period. For their second visit (Visit 2), participants were asked to come fasted to the research center. They were oriented to the study protocol and allowed to rest quietly in a temperature-controlled room for one hour prior to the start of any study procedures. At 13:00, one hour after lunch was served to participants, they were seated in a comfortable chair for the experimental pain testing session. The first two procedures (warmth and heat pain detection thresholds, pressure pain thresholds) were followed by a brief break during which the participants were interviewed by a study nurse and were given the option to opt out of the study if the participants deemed the pain-inducing tests too distressing. After the break, temporal summation and Diffuse Noxious Inhibitory Control (DNIC) were tested. The entire testing battery lasted a maximum of 1.5 hours. The room temperature was set to 25°C at the Research Center testing rooms; nurses adjusted room temperature hourly to maintain temperature continuity and participant comfort. The majority of the pain testing sessions (i.e., 32 out of the 34 sessions) was administered by the same experimenter (JSS), who was blind to the condition of the participant (i.e. insomnia vs. control).
Pain Testing Protocol
Warmth detection threshold (WTh)
-
Pain sensitivity:
Heat pain threshold (HPTh)
Pressure pain threshold (PPTh)
-
Tests assessing pain modulatory mechanisms:
Temporal Summation (TS)
Diffuse Noxious Inhibitory Control (DNIC)
(1) Warmth detection thresholds
(WTh) were assessed using the TSA-II NeuroSensory Analyzer (Medoc, Minneapolis, MS). A Peltier thermode, size 30 × 30 mm2 was secured on the inner palm of the non-dominant hand. From a baseline temperature of 32°C, the thermode was heated at a rate of 0.5°C/sec. Participants were requested to press a control button at the first instant of the sensation of warmth. The stimuli were presented in a train of four with an inter-stimulus interval of 10 seconds. The mean of the response times was calculated to be the detection threshold.
(2) Pain sensitivity
Heat pain thresholds (HPTh) were obtained using the same method described above for WTh, although in this protocol participants were asked to press a control button at the first instant of the sensation of pain (rather than warmth). The stimuli were presented in a train of four at a rate of 0.5°C/sec and inter-stimulus interval of 10 seconds.
Pressure pain thresholds (PPTh) were obtained with use of an electronic pressure algometer (Somedic Sales AB, Hörby, Sweden). Thresholds were assessed at the middle phalanx of the middle and ring finger, following a training session conducted on the index finger. A 1.0cm2 circular probe was positioned on the finger (Brennum et al., 1989) and the pressure was increased at a rate of 30 kPa/sec (cut-off limit was 850 kPa). Participants were instructed to press a control button when they experienced the first sensation of pain. A train of four pressure-pain stimuli were applied at a 15 second intervals and the average of these stimuli was calculated as the pressure pain detection threshold.
(3) Tests involving pain-modulatory mechanisms
Temporal Summation (TS) was assessed with the TSA-II NeuroSensory Analyzer (Medoc, Minneapolis, MS). Using the thermode, four sequences each consisting of ten brief consecutive heat pulses (approximately 0.5 seconds each) with inter-pulse intervals of 2.5 seconds were applied to the non-dominant forearm volar skin. The temperature used to assess TS was tailored to each person’s tolerance level as previously described (Edwards and Fillingim, 2001; Edwards et al., 2003). Specifically, the first test sequence of this procedure had a target temperature of 48°C and an inter-pulse temperature of 42°C. Depending on whether the participant tolerated the initial 10-pulse sequence, the target and inter-pulse temperature of the second sequence was increased or decreased, respectively, by 1.5°C (i.e., increased to 49.5°C with an inter-pulse temperature of 43.5°C or decreased to 46.5°C with an inter-pulse temperature of 40.5°C). In the third sequence, the target and inter-pulse temperatures were again increased or decreased by 1.5°C, depending on whether the participant could tolerate the second sequence or not. The inter-sequence interval was 2 minutes at a temperature of 32°C. The thermode was moved systematically between sequences, starting at the thenar eminance for a practice trial, and sequentially moving cephalad on the volar aspect of the forearm along the innervation of C8-T1 for the remaining three trials, in order to prevent testing on previously stimulated skin areas (see Figure 1).
Figure 1.
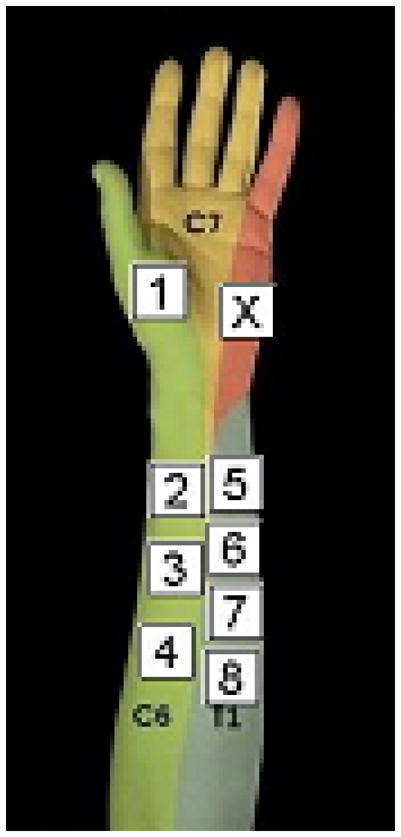
Position of the thermode during pain testing Position X was used for training trials. Position 1 was used for heat pain threshold testing. Position 2, 3, and 4 were used for the three sequences of TS testing. Position 5–8 were used for the four trials of DNIC testing. The thermode was moved in a systematic fashion in order to prevent sensitization or habituation effects due to testing at the same side.
During each test sequence, participants were prompted to rate the intensity of the 1st, 4th, 7th, and 10th thermal pulse using a 0–100 mm visual analogue scale (VAS). A research assistant assisted with the administration of VAS intensity rating scales, which were presented on separate data sheets for each rating. The participant was instructed to say ‘STOP’ as soon as the sensation was no longer tolerable at any point during the testing. Temporal summation of pain is defined as an increase in perceived pain intensity across the 10-pulse sequence, such that the last heat pulse intensity in a sequence is more painful than the first. The time course of pain intensity ratings at highest tolerable temperature was used for statistical analysis.
Diffuse Noxious Inhibitory Control (DNIC)
For this protocol, the test stimulus was the TS sequence at highest tolerable temperature, applied to the outer volar surface of the non-dominant forearm, along C5-6 innervations (see Figure 1). Immersion of the contralateral foot into a hot water bath (47°C) was the conditioning pain stimulus that is intended to activate the pain-inhibitory circuits and thereby decrease the perceived pain of the forearm test stimulus. In total, four DNIC trials were performed: two trials using a hot water bath (47°C) and two trials using a neutral water bath (22°C). The neutral water bath trials served as a distraction-control condition. The trial sequence was applied randomly in the order of hot-neutral-hot-neutral or neutral-hot-neutral-hot, with the order counter-balanced within groups. Techne ® water baths were used (Bibby Scientific US, Burlington, NJ); the hot water bath temperatures were maintained with a clip-on Tempette thermoregulator (TE-10D, Bibby Scientific US, Burlington, NJ)), which heated the water temperature to 47°C, circulated, and controlled water temperature within precise limits. Shortly before each foot immersion, the thermoregulator was removed from the water to comply with hospital safety regulations. Each water bath had a traceable thermometer (Control Company, Friendswood, TX) to assess water temperature throughout foot immersion.
For each trial, the participant’s foot was first submerged in the water bath. After 20 seconds of immersion, the 10-pulse temporal summation sequence was applied to the forearm, and the participant was prompted to rate the pain intensity of the 1st, 4th, 7th, and 10th stimulus using a VAS. After the last stimulus in each series, the participant was prompted to rate the intensity and unpleasantness of the sensation on the foot, before the foot was removed from the water. There was a two-minute rest period between all trials during which the thermode was systematically moved from the distal to proximal sites along the C8-T1 innervated skin to avoid re-stimulation of the previously sensitized skin (see Figure 1).
Spontaneous pain ratings
At bedtime for two weeks prior to the in-laboratory testing, participants documented the intensity of daily spontaneous pain symptoms using a VAS (0 - not experienced at all to 100 -experienced with very high intensity) included on their sleep diaries. The sites of pain included head, joints, muscles, back, and abdomen, as well as two ratings assessing physical discomfort and general body pain. For each pain symptom, pain frequency was calculated by dichotomously coding of daily VAS scores of less than 5 as ‘no pain’ and ratings greater or equal to 5 as ‘pain’. A cut-off value of 5 was chosen to increase discriminative power. Pain frequency was then expressed as the number of days with pain out of the 14-day assessment period for each pain symptom. Pain intensity was calculated by averaging pain intensity ratings across days when pain was present for each item. In addition, the frequency of individual pain symptoms was summed for each study day, in order to calculate global spontaneous pain frequency and intensity scores, respectively.
Statistics
Univariate analyses were used to compare differences between groups for the following output variables: spontaneous pain frequency/intensity, warmth detection threshold [WTh], heat pain threshold [HPTh], and pressure pain threshold [PPTh]). General linear model analysis for repeated measures was used to compare the time course of repeated measured variables (pain intensity ratings in temporal summation sequence) between groups. In the event of an interaction effect (p<0.10), simple contrasts were run to detect which time points differed between groups. PPTh were log transformed before statistical analysis because the data were not normally distributed. Data present mean ±SEM. An alpha value of p<0.05 was considered as significant. An alpha value of p<0.10 was considered as trend towards significance. Data were processed with PASW® Statistics 18 (www.spss.com).
RESULTS
Sample characteristics of primary insomnia and the individually age- and sex- matched healthy control group are shown in Table 1.
Spontaneous pain
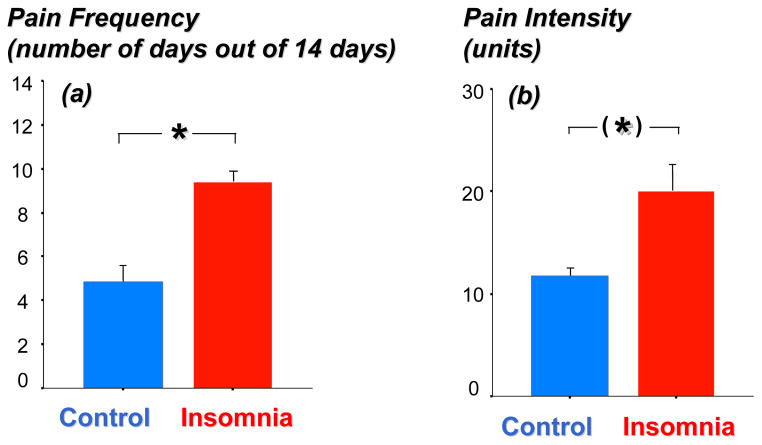
Insomnia subjects reported pain symptoms on twice as many days as healthy controls during the 14-day recording period (9.4±1.0 days in insomnia subjects vs. 4.8±1.1 days in healthy controls; F[1,32]=9.24, p=0.005; see Figure 2a). This effect was mainly due to higher levels of physical discomfort (F[1,32]=6.88, p=0.013) and generalized body pain (F[1,32]=5.15, p=0.030), rather than pain experienced at specific sites, e.g., head, abdomen. Insomnia subjects also rated their pain as more intense than did healthy controls (20±16 vs. 12±7 units in insomnia vs. controls, respectively; F[1,270=3.12, p=0.09; see Figure 2b).
Figure 2.
Depicts the pain frequency (a) and pain intensity (b) across the 14 day recording period in patients with insomnia (N=17) and age- and sex- matched healthy controls (N=17). Pain frequency is based on number of days with reported pain during the measurement period. For pain intensity, sample size is reduced to 16 insomnia patients and 13 healthy controls, as participants reporting no pain experience were excluded from this analysis. Pain variables are comprised of ratings for seven single pain items.
Warmth detection threshold (WTh)
Six matched pairs were excluded due to a technical error. Detection threshold for warmth did not differ between groups (33.5±0.2°C vs. 33.6±0.3°C for insomnia vs. controls, respectively; F[1,20]=0.02, p=0.90), indicating normal perception of non-noxious warmth sensation in insomnia subjects.
Pain sensitivity
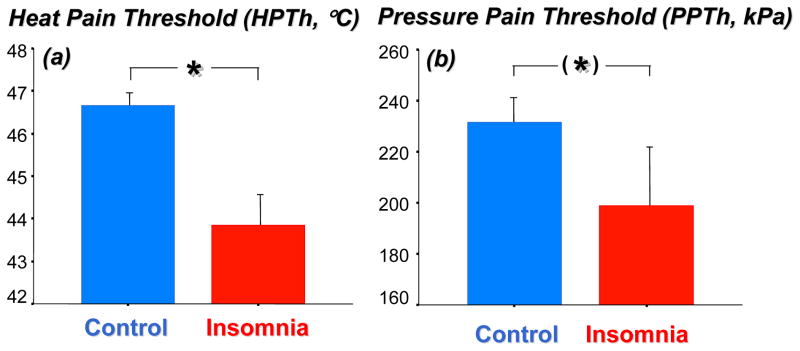
Heat pain thresholds (HPTh) were on average 3.5°C lower in subjects suffering from insomnia compared to matched healthy controls (p<0.05, see Figure 3a).
Figure 3.
(a) Heat pain threshold (HPTh,°C) and (b) pressure pain threshold (PPTh, kPa) in patients with insomnia (N=17) and age-and sex-matched healthy controls (N=17). F[1,32]=7.70, p=0.009, for HPTh; F[1,32]=3.30, p<0.08 for PPTh.
Pressure pain thresholds (PPTh) trended to be significantly lower in patients suffering from insomnia than healthy controls (p=0.08, see Figure 3b).
Tests assessing pain-modulatory mechanisms
Temporal summation (TS)
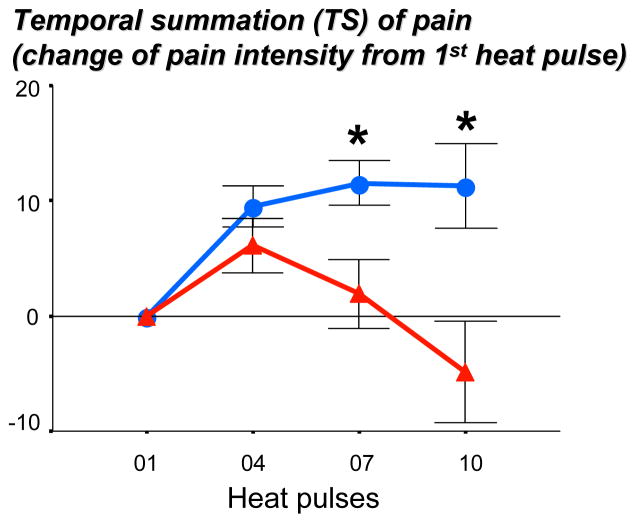
The highest tolerable temperature used with the repeated heat pulse sequence did not significantly differ between insomnia subjects and healthy controls (48.9±0.30°C vs. 49.5±0.34°C; F[1,32]=1.91, p=0.18). However, the intensity ratings of the heat pulse sequence showed a significant interaction effect (F[3,96]=5.21, p=0.01, see Figure 4). While pain intensity ratings initially increased in both groups, ratings of the control group continued to increase while ratings in the insomnia group rapidly declined and dropped below baseline value at the last measurement point. These findings indicate that insomnia patients show a decreased, rather that the predicted increased, TS of pain.
Figure 4.
Pain intensity ratings of a 10-heat pulse sequence at highest tolerable temperature in patients with primary insomnia (N=17, blue line) and age-and sex-matched healthy controls (N=17, red line). Perceived intensity differed significantly between groups (F[3,96]=5.21, p=0.01 for interaction effect), with significant differences between pulse 7 (F[1,31]=5.67, p<0.05) and pulse 10 (F[1,31]=6.33, p<0.02). Data are presented as differences from pulse 1 for better visibility.
Diffuse noxious inhibitory control (DNIC)
Conditioning stimuli (hot and neutral water bath)
The pain intensity ratings of the hot vs. neutral water bath were 75±4 vs. 31±6 units in insomnia subjects, which was similar to controls (71±4 vs. 20±4 units, F[1,32]=0.78, p>0.05 for interaction effect). As expected, there was a significant effect of hot vs. neutral water temperatures (F[1,32]=180.35, p<0.001 in the total sample). Similarly, the unpleasantness ratings of the hot vs. neutral water bath were similar in insomnia and controls (65±6 vs. 20±6 units in insomnia participants and 61±6 vs. 7±3 units in control participants, F[1,32]=0.52. p>0.05 for interaction effect), with significant effect of hot vs. neutral water temperatures (F[1,32]=117.12, p<0.001 in the total sample). Change of water temperature between the time of foot entry and exit from the water bath did not significantly differ between groups in either water temperature condition (neutral water bath, p=0.07, hot water bath, p=0.68). Thus, intensity and unpleasantness ratings, as well as change of water bath temperatures did not differ between insomnia and controls and therefore do not confound ratings of the test stimuli.
Test stimuli (TS sequence)
Six out of 17 insomnia subjects (35%) and 3 out of 17 control subjects (18%) had their initial temperature tailored to the individual pain tolerance levels adjusted again (e.g., reduced by 1.5°C) after reporting intolerable/unsustainable pain levels during the first trial.
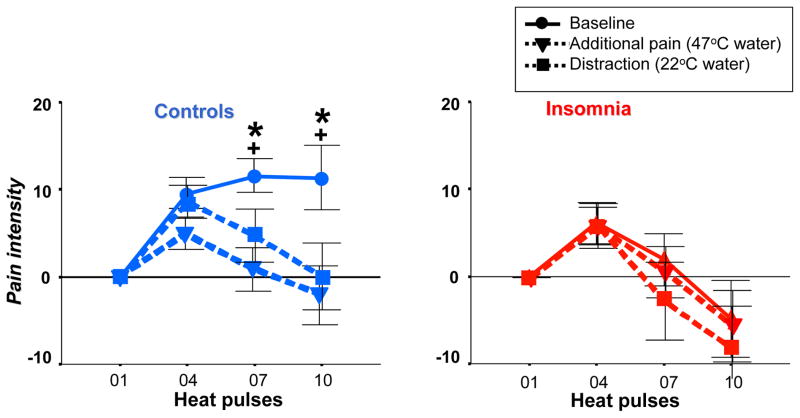
As shown in Figure 5 (left panel), healthy controls reported the 10-heat pulse TS sequence as significantly less painful during exposure to conditional heat pain of the hot water bath and the conditional distraction stimulus of the neutral water bath, compared to baseline (i.e. TS sequence without additional exposure to conditional heat pain/distraction). This result reflects physiological activation of endogenous pain-inhibitory circuits that are mediated by heat pain, distraction or both stimuli. In contrast, insomnia subjects reported no decreases in pain ratings during either exposure to the conditional heat pain or distraction neutral temperature stimulus, compared to baseline. This result implies a failure to mount endogenous pain inhibition (Figure 5, right panel).
Figure 5.
Pain intensity ratings of a 10-heat pulse sequence under (●) baseline condition, (▼) additional pain condition (i.e., foot immersion in hot water bath at 47 °C), and (■) distraction condition (i.e., foot immersion into neutral water bath at 22 °C). Data are presented as change from first heat pulse for better visibility as no differences were observed for intensity ratings of first heat pulse between groups. Left panel: In controls, both the additional pain and the distraction condition lead to a pain-inhibitory effect when compared to baseline (F[3, 48] > 5.8, p < 0.02 for interaction effects). Additional pain and distraction conditions did not differ (F[3, 48] = 1.33, p = 0.28 for interaction). Right panel: In primary insomnia, neither the additional pain nor the distraction condition leads to a pain-inhibitory effect when compared to baseline (F[3, 48] < 0.45, p > 0.67 for interaction effects. * and + represent significant differences between baseline and the additional pain or distraction conditions, respectively (p < .05 for both).
It is of note that the pain intensity ratings and heat pulse response curve (Figure 5) during the baseline TS sequence in insomnia participants resembles the curves observed in healthy controls during either conditional heat pain or distraction temperature challenges. This suggests that maximum recruitment of pain-inhibitory circuits was already present at baseline, as no further response mounting occurred during conditional heat pain or distraction challenge during DNIC testing.
DISCUSSION
The current data provide the first empirical evidence that individuals with primary insomnia show abnormalities in pain processing. Specifically, this study demonstrates that individuals with primary insomnia experienced spontaneous pain more frequently and intensely, have a higher sensitivity to evoked heat and pressure pain, and have a dysfunctional pain inhibition system compared to healthy individuals. These findings support the overall hypothesis that insufficient sleep, here observed in the form of insomnia, may contribute to the development or amplification of pain independent from the influence of medication or other medical disorders. This is of critical importance considering that insomnia is a very common form of sleep disturbance, with a lifetime prevalence of 25–33% (for review, see Pigeon, 2010).
Spontaneous pain reporting
Cross-sectional and longitudinal studies have consistently reported that insufficient sleep and the subjective reporting of pain are interrelated. This association has been shown in the general and various clinical pain populations, as well as in experimental studies where one or more days of total or partial sleep deprivation led to an increase in the experience of spontaneous pain (see introduction). Experimental studies have typically tested the effects of a more drastic, consistent decrease in sleep quality of quantity over a short period of time. In contrast, however, patients suffering from insomnia, typically experience large day-to-day variability in both sleep duration and quality (Buysse et al., 2010). Thus, the current study finding supports the hypothesis that the specific types of insufficient sleep in primary insomnia are likely to contribute to, or amplify, the experience of spontaneous pain.
Pain sensitivity
To our knowledge, pain sensitivity has not previously been assessed in patients suffering from primary insomnia independent from the influence of psychiatric or pain-related disorders. We report here that these patients have significantly lower heat pain thresholds (by almost 3°C) and demonstrated a trend towards lower pressure pain thresholds. These changes were not due to a general change in somatosensory processing, as warmth detection thresholds were similar in insomnia and control participants. Similarly, TMD patients with an additional diagnosis of primary insomnia have been reported to have increased pain sensitivity to pressure and heat pain stimuli compared to those without a sleep disorder (Smith et al., 2009). There is also strong evidence from experimental studies that insufficient sleep itself is able to increase pain sensitivity (see introduction). The current study findings support that, beyond the experimental manipulation of sleep duration and continuity, the type of sleep disturbances experienced in primary insomnia contribute to an increase in pain sensitivity. At this point, it is not clear which aspects of sleep in primary insomnia are predictive of changes in pain sensitivity, such as duration, fragmentation, or even effects on daytime functioning (e.g., subjective sleepiness, which has been recently found related to pain sensitivity (Chhangani et al., 2009)).
Pain-modulatory mechanisms
Findings from testing pain facilitation and pain inhibition, as assessed by temporal summation and DNIC, respectively, provide preliminary data that contributes to our understanding of the physiological mechanisms underlying central pain processing abnormalities in primary insomnia. While the temporal summation of heat pain response increased in healthy controls as has been observed previously, the responses in primary insomnia subjects unexpectedly declined to a level below baseline after an initial brief rise (Figure 4).
Temporal summation reflects a facilitation of the spinal neuronal response to repeated C fiber stimulation (Mendell, 1966), and is often used as an index of the sensitizability of central pain transmission neurons (Herrero et al., 2000). Stimuli used in the temporal summation sequence can also be strong enough to activate pain inhibitory circuits, thereby counteracting pain facilitation and leading to less active temporal summation (Gozariu et al., 1997). These complementary actions of opposing pain-facilitatory and pain-inhibitory effects have also been observed during the development of inflammation. In experimental models of arthritis, for example, pain facilitation (e.g., spinal cord hyperexcitability evoked by inflammation) is counteracted by an increase in the effectiveness of descending inhibition (Schaible and Grubb, 1993).
With this in mind, our findings of less temporal summation in insomnia suggest that pain-inhibitory circuits are in constant activation, thereby counteracting the pain-facilitatory response (i.e. TS of pain) in this sample to a greater extent than is observed in healthy participants. Constant activation of the descending pain inhibition in insomnia may be the compensatory response to continually enhanced or amplified noxious input, as indicated by our findings of increased spontaneous pain and pain sensitivity in insomnia. Without this continuous counter-regulatory (pain inhibitory) effort, insomnia patients may have experienced even more spontaneous pain and have higher sensitivity to pain than was observed in this study.
While findings from the temporal summation test suggest that the pain inhibition system in patients with primary insomnia is in a constantly activated state, this system failed to respond when directly challenged using the DNIC paradigm. While healthy control participants in the current study showed the expected pain-inhibitory effect under conditions of conditional heat pain (hot water), as well as distraction (neutral water), participants with primary insomnia were unable to inhibit pain under either of these conditions. Distraction is an effective stimulus to diminish pain and frequently used as a pain coping strategy (Keogh et al., 2000), and while it has often been questioned whether pain inhibition in response to an additional painful stimuli is simply due to a distraction process, recent evidence supports that the mechanisms through which additional pain or distraction inhibit pain are largely separable. (Moont et al., 2010; Lautenbacher et al., 2007). Thus, our findings indicate that the unresponsiveness of the pain-inhibitory system when directly challenged is independent of the stimulus used to trigger the system (e.g., pain or distraction).
Our findings of a diminished response of descending pain inhibition to DNIC testing in the context of attenuated temporal summation of pain, elevated rates of spontaneous pain, and increased pain sensitivity in primary insomnia participants lead us to hypothesize that pain-inhibitory circuits are in a state of constant activation in order to compensate for ongoing subclinical pain, which ultimately leads to a ceiling effect, i.e., a state of maximal activation of the pain inhibition system. As a result, both pain facilitation and inhibition functions, as quantified here by temporal summation and DNIC testing, respectively, are abnormal in participants with primary insomnia. While supported by our data, this hypothesis is preliminary and will require longitudinal testing of pain symptoms and regulatory functions in a sample of participants with new onset insomnia.
This hypothesized maximal activation of the pain inhibitory system may have several consequences. For example, various analgesic medications that are known to reduce pain, in part through activation of pain-inhibitory circuits, may not be as effective in primary insomnia populations. These medications include commonly used non-steroidal anti-inflammatory drugs (NSAIDS, (Rady et al., 2001), as well as opioids (Millan, 2002). Further, we suggest that the constantly activated pain-inhibitory circuits we propose in our young participants may eventually exhaust over the course of chronic insomnia. This transition may result in a dis-inhibition of pain processing and contribute to the transition from acute pain symptoms to chronic pain conditions, which are high co-morbid disorders in insomnia populations.
Acknowledgments
This study was funded by an investigator-initiated grant from Sepracor Inc and Grant Number UL1 RR025758 and M01-RR-01032 from the National Center for Research Resources to the Harvard Clinical and Translational Science Center.
References
- Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. DSM-IV TR. 4. Arlington, VA: 2000. text revision. [Google Scholar]
- Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurements of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Medicine. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chhangani BS, Roehrs TA, Harris EJ, Hyde M, Drake C, Hudgel DW, Roth T. Pain Sensitivity in Sleepy Pain-Free Normals. Sleep. 2009;32:1011–1017. [PMC free article] [PubMed] [Google Scholar]
- Cooperman NR, Mullin FJ, Kleitman N. Studies on the physiology of sleep. XI. Further observations on the effects of prolonged sleeplessness. Am J Physiol. 1934;107:589–593. [Google Scholar]
- Edwards RR, Fillingim RB. Effects of age on temporal summation and habituation of thermal pain: Clinical relevance in healthy older and younger adults. Journal of Pain. 2001;2:307–317. doi: 10.1054/jpai.2001.25525. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB, Ness TJ. Age-related differences in endogenous pain modulation: a comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Pain. 2003;101:155–165. doi: 10.1016/s0304-3959(02)00324-x. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Grace E, Peterson S, Klick B, Haythornthwaite JA, Smith MT. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. European Journal of Pain. 2009;13:1043–1047. doi: 10.1016/j.ejpain.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. Structured clinical interview for DSM-IV axis I disorders (SCID-I) 2004. [DOI] [PubMed] [Google Scholar]
- Gozariu M, Bragard D, Willer JC, Le Bars D. Temporal summation of C-fiber afferent inputs: Competition between facilitatory and inhibitory effects on C-fiber reflex in the rat. J Neurophysiol. 1997;78:3165–3179. doi: 10.1152/jn.1997.78.6.3165. [DOI] [PubMed] [Google Scholar]
- Haack M, Mullington JM. Sustained sleep restriction reduces emotional and physical well-being. Pain. 2005;119:56–64. doi: 10.1016/j.pain.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145–1152. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack M, Scott-Sutherland J, Sethna N, Mullington JM. Mechanisms of sleep loss-pain interactions. In: Lavigne G, Cistulli PA, Smith MT, editors. Sleep medicine for dentists. A practical overview. Hanover Park, IL: Quintessence Publishing Co; 2009. pp. 155–160. [Google Scholar]
- Herrero JF, Laird JMA, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Keogh E, Hatton K, Ellery D. Avoidance versus focused attention and the perception of pain: differential effects for men and women. Pain. 2000;85:225–230. doi: 10.1016/s0304-3959(99)00270-5. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundermann B, Spernal J, Huber MT, Krieg JC, Lautenbacher S. Sleep deprivation affects thermal pain thresholds but not somatosensory thresholds in healthy volunteers. Psychosom Med. 2004;66:932–937. doi: 10.1097/01.psy.0000145912.24553.c0. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Prager M, Rollman GB. Pain additivity, diffuse noxious inhibitory controls, and attention: A functional measurement analysis. Somatosensory and Motor Research. 2007;24:189–201. doi: 10.1080/08990220701637638. [DOI] [PubMed] [Google Scholar]
- Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). 1. Effects on dorsal horn convergent neurons in the rat. Pain. 1979;6:283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, Helfgott SM, Solomon DH, Clauw DJ, Karlson EW. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Research and Therapy. 2009;11 doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. Physiologial properties of unmyelinated fiber projection to spinal cord. Exp Neurol. 1966;16:316. doi: 10.1016/0014-4886(66)90068-9. [DOI] [PubMed] [Google Scholar]
- Milanov I, Bogdanova D. Trigemino-cervical reflex in patients with headache. Cephalalgia. 2003;23:35–38. doi: 10.1046/j.1468-2982.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. ‘Pain inhibits pain’ mechanisms: Is pain modulation simply due to distraction? Pain. 2010;150:113–20. doi: 10.1016/j.pain.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Older SA, Battafarano DF, Danning CL, Ward JA, Grady EP, Derman S, Russell IJ. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–1186. [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Pigeon WR. Diagnosis, prevalence, pathways, consequences and treatment of insomnia. Indian Journal of Medical Research. 2010;131:321–332. [PMC free article] [PubMed] [Google Scholar]
- Pud D, Granovsky Y, Yarnitsky D. The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain. 2009;144:16–19. doi: 10.1016/j.pain.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Rady JJ, Campbell WB, Fujimoto JM. Antianalgesic action of nociceptin originating in the brain is mediated by spinal prostaglandin E-2 in mice. Journal of Pharmacology and Experimental Therapeutics. 2001;296:7–14. [PubMed] [Google Scholar]
- Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Grubb BD. Afferent and spinal mechanisms of joint pain. Pain. 1993;55:5–54. doi: 10.1016/0304-3959(93)90183-P. [DOI] [PubMed] [Google Scholar]
- Schramm E, Hohagen F, Grasshoff U, Riemann D, Hajak G, Weess HG, Berger M. Test-retest reliability and validity of the structured interview for sleep disorders according to DSM--III-R. Am J Psychiatry. 1993;150:867–872. doi: 10.1176/ajp.150.6.867. [DOI] [PubMed] [Google Scholar]
- Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- Smith MT, Wickwire EM, Grace EG, Edwards RR, Buenaver LF, Peterson S, Klick B, Haythornthwaite JA. Sleep Disorders and their Association with Laboratory Pain Sensitivity in Temporomandibular Joint Disorder. Sleep. 2009;32:779–790. doi: 10.1093/sleep/32.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk G, Veldhuijzen DS. Perspective on diffuse noxious inhibitory controls as a model of endogenous pain modulation in clinical pain syndromes. Journal of Pain. 2010;11:408–419. doi: 10.1016/j.jpain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Dewey JE. SF-36 Health Survey®. Manual and interpretation guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]