Table 1.
Ring expansion of various 1-sulfonyl 4-tertiary alcohol triazoles 3 yielding products 5. General reaction conditions: 1-sulfonyl triazole (1.0 mmol, 1.0 equiv.), rhodium(II) octanoate (0.5 mol%), CHCl3 (2.0 mL), 70 °C, mw, 5 – 60 min. All values shown represent isolated yields.
| Entry | Triazole | [°C/min] | Product | Yield |
|---|---|---|---|---|
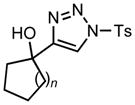
|
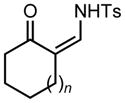
|
|||
| 1 | 3a: n = 1 | 70/15 | 5a: n = 1 | 91% |
| 2 | 3b: n = 2 | 70/15 | 5b: n = 2 | 93% |
| 3 | 3c: n = 3 | 70/15 | 5c: n = 3 | 94% |
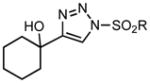
|
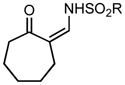
|
|||
| 4 | 3d: R = p-OMePh | 70/60 | 5d: R = p-OMePh | 95% |
| 5 | 3e: R = p-IPh | 70/5 | 5e: R = p-IPh | 71% |
| 6 | 3f: R = iPr | 70/20 | 5f: R = iPr | 66% |
| 7 |
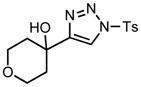 3g |
70/30 |
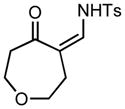 5g |
92% |
| 8 |
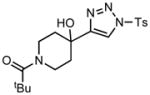 3h |
70/45 |
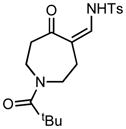 5h |
98% |
| 9 |
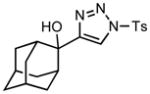 3i |
70/60 |
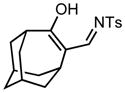 5i |
89% |
| 10 |
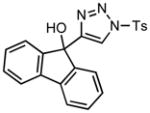 3j |
70/30 |
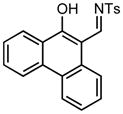 5j |
95% |
