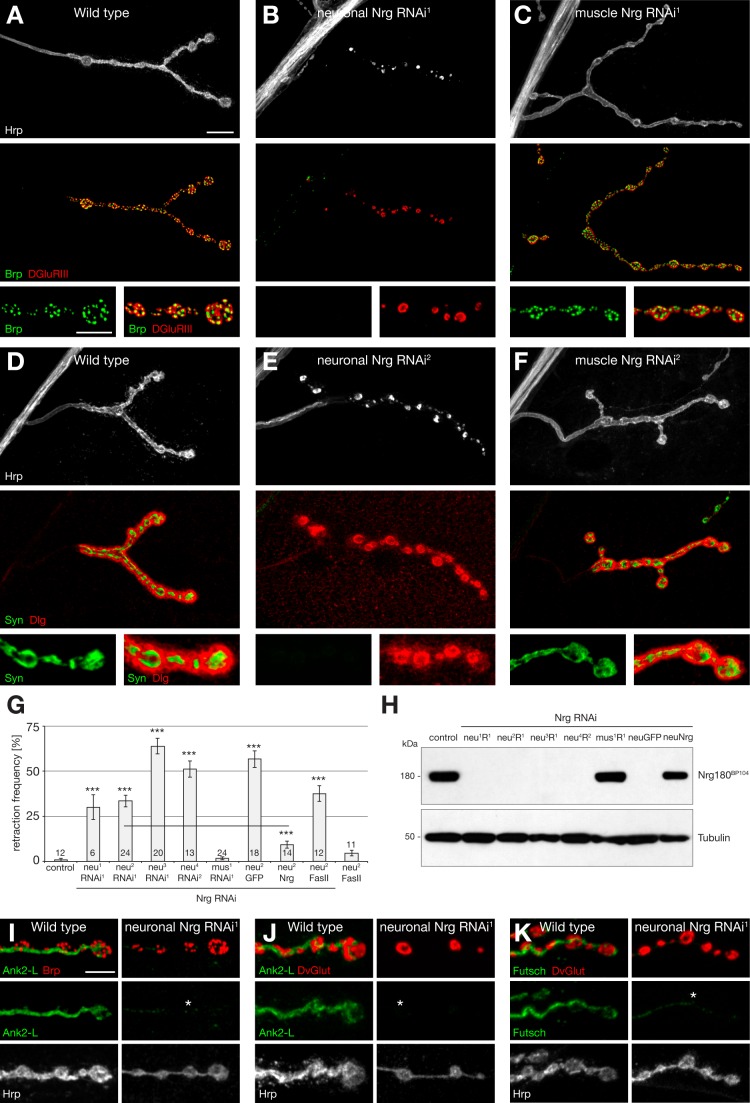
Figure 1. Presynaptic Nrg is essential for synapse stability.
(A–C) NMJs on muscle 4 stained for the presynaptic motoneuron membrane (Hrp, white), the presynaptic active zone marker Brp (green), and postsynaptic glutamate receptors (DGluRIII, red). (A) A stable wild-type NMJ indicated by perfect apposition of pre- and postsynaptic markers. (B) Knockdown of presynaptic Nrg resulted in severe synaptic retraction indicated by the loss of presynaptic Brp despite the presence of postsynaptic glutamate receptors and by a fragmentation of the presynaptic membrane. Synaptic retractions caused a characteristic fusion of postsynaptic glutamate receptor clusters. (C) Knockdown of postsynaptic Nrg did not impair synapse stability. (D–F) NMJs on muscle 4 stained for the presynaptic motoneuron membrane (Hrp, white), presynaptic vesicles (Syn, green), and postsynaptic Dlg (red). Identical phenotypes were observed when using an independent Nrg RNAi line and independent pre- and postsynaptic markers. Only presynaptic knockdown of Nrg resulted in synaptic retractions indicated by a selective loss of synaptic vesicles and a fragmentation of the presynaptic membrane. (G) Quantification of synaptic retractions. Neuronal but not muscle-specific knockdown of Nrg using different Gal4 driver combinations or independent RNAi constructs resulted in a significant increase in synaptic retractions on muscle 4. The synaptic retraction frequency was significantly rescued (p≤0.001) by co-expression of UAS–nrg180 but not when we co-expressed either UAS–mCD8–GFP or UAS–fasII. Neuronal expression of UAS–fasII in a wild-type background (neu2 FasII) did not result in a significant increase in synaptic retractions (genotypes: neu1 = elavC155–Gal4; neu2 = elavC155–Gal4; ok371–Gal4; neu3 = elavC155–Gal4; UAS-dcr2; neu4 = elavC155–Gal4; sca–Gal4 UAS–dcr2; mus1 = UAS–dcr2; mef2–Gal4; RNAi1 = VDRC6688; RNAi2 = VDRC107991; GFP, Nrg, and FasII indicate co-expression of the corresponding UAS construct; the number of analyzed animals is indicated). (H) Western blot analysis of the genotypes in (G) probed with an antibody against Nrg180 (Nrg180BP104). Neuronal but not muscle-specific Nrg RNAi resulted in efficient knockdown of Nrg180 in larval brains. Nrg180 levels could be rescued by co-expression of Nrg180 but not by co-expression of mCD8-GFP. (I–K) Characterization of multiple presynaptic markers after knockdown of presynaptic Nrg. (I) In wild-type animals presynaptic Ank2-L (green) and Brp (red) were present in all synaptic boutons. In the absence of Nrg, Ank2-L was lost prior to Brp at distal parts of an NMJ that was still stable as indicated by the continuous membrane staining. (J) Similarly, Ank2-L was lost prior to the presynaptic vesicle marker DvGlut (red) at a semistable NMJ. (K) In wild-type animals the microtubule-associated protein Futsch (green) and DvGlut (red) were present in all boutons. Knockdown of Nrg resulted in a loss of Futsch prior to the disassembly of DvGlut at early stages of synapse retraction. Scale bar in (A) corresponds to (A–F), 10 µm, inset 5 µm. Scale bar in (I) corresponds to (I–K), 5 µm. Error bars represent SEM.