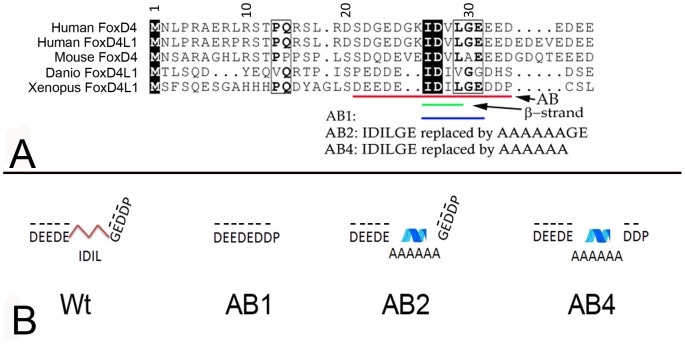
Figure 8. Conserved amino acids in the Acidic Blob region of FoxD4/FoxD4L1 proteins that were mutated for this study.
(A) CLUSTALW alignment of the N-terminal region including the Acid Blob (AB, denoted by red line), as in Figure 3. The highly conserved IDIL sequence is predicted to form a short β-strand (green line). Six amino acids, denoted by the blue line, were deleted in the AB1 construct. The amino acid substitutions made in the AB2 and AB4 constructs are noted. (B) Predicted protein folding within the Acidic Blob of the wild-type (Wt) and AB mutated Xenopus FoxD4L1 proteins. Red lines denote the short β-strand, and the blue ribbon denotes a 1.7 turn α-helix predicted to form by the 6 alanine residues. Dashes over the aspartic (D) and glutamic (E) acid residues indicate negative charges.