Abstract
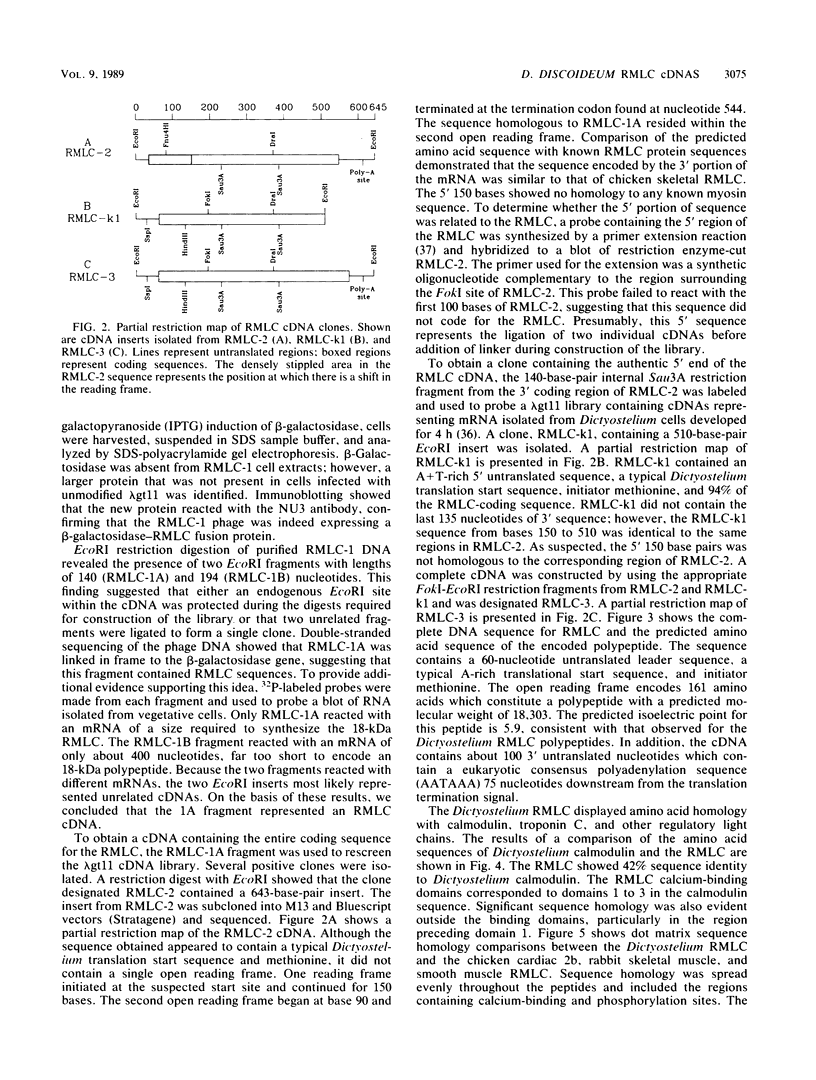
Phosphorylation of the regulatory light chains (RMLC) of nonmuscle myosin can increase the actin-activated ATPase activity and filament formation. Little is known about these regulatory mechanisms and how the RMLC are involved in ATP hydrolysis. To better characterize the nonmuscle RMLC, we isolated cDNAs encoding the Dictyostelium RMLC. Using an antibody specific for the RMLC, we screened a lambda gt11 expression library and obtained a 200-base-pair clone that encoded a portion of the RMLC. The remainder of the sequence was obtained from two clones identified by DNA hybridization, using the 200-base-pair cDNA. The composite RMLC cDNA was 645 nucleotides long. It contained 60 base pairs of 5' untranslated, 483 bases of coding, and 102 base pairs of 3' untranslated sequence. The amino acid sequence predicted an 18,300-dalton protein that shares 42% amino acid identity with Dictyostelium calmodulin and 30% identity with the chicken skeletal myosin RMLC. This sequence contained three regions that were similar to the E-F hand calcium-binding domains found in calmodulin, troponin C, and other myosin light chains. A sequence similar to the phosphorylation sequence found in chicken gizzard and skeletal myosin light chains was found at the amino terminus. Genomic Southern blot analysis suggested that the Dictyostelium genome contains a single gene encoding the RMLC. Analysis of RMLC expression patterns during Dictyostelium development indicated that accumulation of this mRNA increases just before aggregation and again during culmination. This pattern is similar to that obtained for the Dictyostelium essential myosin light chain and suggests that expression of the two light chains is coordinated during development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Conti M. A. Phosphorylation of platelet myosin increases actin-activated myosin ATPase activity. Nature. 1975 Aug 14;256(5518):597–598. doi: 10.1038/256597a0. [DOI] [PubMed] [Google Scholar]
- Berlot C. H., Devreotes P. N., Spudich J. A. Chemoattractant-elicited increases in Dictyostelium myosin phosphorylation are due to changes in myosin localization and increases in kinase activity. J Biol Chem. 1987 Mar 15;262(8):3918–3926. [PubMed] [Google Scholar]
- Bourguignon L. Y., Nagpal M. L., Hsing Y. C. Phosphorylation of myosin light chain during capping of mouse T-lymphoma cells. J Cell Biol. 1981 Dec;91(3 Pt 1):889–894. doi: 10.1083/jcb.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carboni J. M., Condeelis J. S. Ligand-induced changes in the location of actin, myosin, 95K (alpha-actinin), and 120K protein in amebae of Dictyostelium discoideum. J Cell Biol. 1985 Jun;100(6):1884–1893. doi: 10.1083/jcb.100.6.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Chisholm R. L., Rushforth A. M., Pollenz R. S., Kuczmarski E. R., Tafuri S. R. Dictyostelium discoideum myosin: isolation and characterization of cDNAs encoding the essential light chain. Mol Cell Biol. 1988 Feb;8(2):794–801. doi: 10.1128/mcb.8.2.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. Isolation and characterization of myosin from amoebae of Dictyostelium discoideum. J Mol Biol. 1974 Jun 25;86(2):209–222. doi: 10.1016/0022-2836(74)90013-8. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Côté G. P., Albanesi J. P., Ueno T., Hammer J. A., 3rd, Korn E. D. Purification from Dictyostelium discoideum of a low-molecular-weight myosin that resembles myosin I from Acanthamoeba castellanii. J Biol Chem. 1985 Apr 25;260(8):4543–4546. [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Holmsen H. Myosin phosphorylation in intact platelets. J Biol Chem. 1981 Jul 25;256(14):7510–7514. [PubMed] [Google Scholar]
- Daniel J. L., Molish I. R., Rigmaiden M., Stewart G. Evidence for a role of myosin phosphorylation in the initiation of the platelet shape change response. J Biol Chem. 1984 Aug 10;259(15):9826–9831. [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith L. M., Downs S. M., Spudich J. A. Myosin light chain kinase and myosin light chain phosphatase from Dictyostelium: effects of reversible phosphorylation on myosin structure and function. J Cell Biol. 1987 May;104(5):1309–1323. doi: 10.1083/jcb.104.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Rodgers M. E. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., House C. Phosphorylation of a synthetic heptadecapeptide by smooth muscle myosin light chain kinase. J Biol Chem. 1982 Nov 25;257(22):13349–13353. [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., House C. Role of basic residues in the phosphorylation of synthetic peptides by myosin light chain kinase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7471–7475. doi: 10.1073/pnas.80.24.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B. Spatial requirements for location of basic residues in peptide substrates for smooth muscle myosin light chain kinase. J Biol Chem. 1985 Mar 25;260(6):3355–3359. [PubMed] [Google Scholar]
- Kerrick W. G., Bourguignon L. Y. Regulation of receptor capping in mouse lymphoma T cells by Ca2+-activated myosin light chain kinase. Proc Natl Acad Sci U S A. 1984 Jan;81(1):165–169. doi: 10.1073/pnas.81.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Sequence organization in Dictyostelium: unique structure at the 5'-ends of protein coding genes. Nucleic Acids Res. 1983 Jan 25;11(2):541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Developmental consequences of the lack of myosin heavy chain in Dictyostelium discoideum. Dev Biol. 1988 Jul;128(1):178–184. doi: 10.1016/0012-1606(88)90280-1. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R. Partial purification of two myosin heavy chain kinases from Dictyostelium discoideum. J Muscle Res Cell Motil. 1986 Dec;7(6):501–509. doi: 10.1007/BF01753566. [DOI] [PubMed] [Google Scholar]
- Kuczmarski E. R., Spudich J. A. Regulation of myosin self-assembly: phosphorylation of Dictyostelium heavy chain inhibits formation of thick filaments. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7292–7296. doi: 10.1073/pnas.77.12.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar C. C., Cribbs L., Delaney P., Chien K. R., Siddiqui M. A. Heart myosin light chain 2 gene. Nucleotide sequence of full length cDNA and expression in normal and hypertensive rat. J Biol Chem. 1986 Feb 25;261(6):2866–2872. [PubMed] [Google Scholar]
- Kuznicki J., Baryłko B. Phosphorylation of myosin in smooth muscle and non-muscle cells. In vitro and in vivo effects. Int J Biochem. 1988;20(6):559–568. doi: 10.1016/0020-711x(88)90094-8. [DOI] [PubMed] [Google Scholar]
- Lacombe M. L., Podgorski G. J., Franke J., Kessin R. H. Molecular cloning and developmental expression of the cyclic nucleotide phosphodiesterase gene of Dictyostelium discoideum. J Biol Chem. 1986 Dec 25;261(36):16811–16817. [PubMed] [Google Scholar]
- Maruta H., Baltes W., Dieter P., Marmé D., Gerisch G. Myosin heavy chain kinase inactivated by Ca2+/calmodulin from aggregating cells of Dictyostelium discoideum. EMBO J. 1983;2(4):535–542. doi: 10.1002/j.1460-2075.1983.tb01459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta H., Gadasi H., Collins J. H., Korn E. D. The isolated heavy chain of an Acanthamoeba myosin contains full enzymatic activity. J Biol Chem. 1978 Sep 25;253(18):6297–6300. [PubMed] [Google Scholar]
- Nellen W., Silan C., Firtel R. A. DNA-mediated transformation in Dictyostelium discoideum: regulated expression of an actin gene fusion. Mol Cell Biol. 1984 Dec;4(12):2890–2898. doi: 10.1128/mcb.4.12.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto Y., Sekine T., Grammer J., Yount R. G. The essential light chains constitute part of the active site of smooth muscle myosin. Nature. 1986 Nov 6;324(6092):78–80. doi: 10.1038/324078a0. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Misconi L. Y., Kemp B. E. Smooth muscle myosin kinase requires residues on the COOH-terminal side of the phosphorylation site. Peptide inhibitors. J Biol Chem. 1986 Jan 5;261(1):25–27. [PubMed] [Google Scholar]
- Persechini A., Hartshorne D. J. Phosphorylation of smooth muscle myosin: evidence for cooperativity between the myosin heads. Science. 1981 Sep 18;213(4514):1383–1385. doi: 10.1126/science.6455737. [DOI] [PubMed] [Google Scholar]
- Peters D. J., Knecht D. A., Loomis W. F., De Lozanne A., Spudich J., Van Haastert P. J. Signal transduction, chemotaxis, and cell aggregation in Dictyostelium discoideum cells without myosin heavy chain. Dev Biol. 1988 Jul;128(1):158–163. doi: 10.1016/0012-1606(88)90278-3. [DOI] [PubMed] [Google Scholar]
- Prince H. P., Trayer H. R., Henry G. D., Trayer I. P., Dalgarno D. C., Levine B. A., Cary P. D., Turner C. Proton nuclear-magnetic-resonance spectroscopy of myosin subfragment 1 isoenzymes. Eur J Biochem. 1981 Dec;121(1):213–219. doi: 10.1111/j.1432-1033.1981.tb06451.x. [DOI] [PubMed] [Google Scholar]
- Pustell J., Kafatos F. C. A convenient and adaptable package of computer programs for DNA and protein sequence management, analysis and homology determination. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):643–655. doi: 10.1093/nar/12.1part2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Cell division: direct measurement of maximum tension exerted by furrow of echinoderm eggs. Science. 1967 Jun 2;156(3779):1241–1243. doi: 10.1126/science.156.3779.1241. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliwa M. Mechanisms of intracellular organelle transport. Cell Muscle Motil. 1984;5:1-82,403-6. doi: 10.1007/978-1-4684-4592-3_1. [DOI] [PubMed] [Google Scholar]
- Scholey J. M., Smith R. C., Drenckhahn D., Groschel-Stewart U., Kendrick-Jones J. Thymus myosin. Isolation and characterization of myosin from calf thymus and thymic lymphocytes, and studies on the effect of phosphorylation of its Mr = 20,000 light chain. J Biol Chem. 1982 Jul 10;257(13):7737–7745. [PubMed] [Google Scholar]
- Schroeder T. E. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1688–1692. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder T. E. The contractile ring. II. Determining its brief existence, volumetric changes, and vital role in cleaving Arbacia eggs. J Cell Biol. 1972 May;53(2):419–434. doi: 10.1083/jcb.53.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan M., Burke M. The free heavy chain of vertebrate skeletal myosin subfragment 1 shows full enzymatic activity. J Biol Chem. 1982 Jan 25;257(2):1102–1105. [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J Biol Chem. 1974 Sep 25;249(18):6013–6020. [PubMed] [Google Scholar]
- Vaillancourt J. P., Lyons C., Côté G. P. Identification of two phosphorylated threonines in the tail region of Dictyostelium myosin II. J Biol Chem. 1988 Jul 25;263(21):10082–10087. [PubMed] [Google Scholar]
- Varnum B., Soll D. R. Effects of cAMP on single cell motility in Dictyostelium. J Cell Biol. 1984 Sep;99(3):1151–1155. doi: 10.1083/jcb.99.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Hydrolysis of ATP and reversible binding to F-actin by myosin heavy chains free of all light chains. Nature. 1981 Aug 6;292(5823):560–562. doi: 10.1038/292560a0. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., De Lozanne A., Leinwand L. A., Spudich J. A. Conserved protein domains in a myosin heavy chain gene from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9433–9437. doi: 10.1073/pnas.83.24.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Wessels D., Soll D. R., Knecht D., Loomis W. F., De Lozanne A., Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988 Jul;128(1):164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Witke W., Nellen W., Noegel A. Homologous recombination in the Dictyostelium alpha-actinin gene leads to an altered mRNA and lack of the protein. EMBO J. 1987 Dec 20;6(13):4143–4148. doi: 10.1002/j.1460-2075.1987.tb02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumura S., Fukui Y. Reversible cyclic AMP-dependent change in distribution of myosin thick filaments in Dictyostelium. Nature. 1985 Mar 14;314(6007):194–196. doi: 10.1038/314194a0. [DOI] [PubMed] [Google Scholar]
- Yumura S., Mori H., Fukui Y. Localization of actin and myosin for the study of ameboid movement in Dictyostelium using improved immunofluorescence. J Cell Biol. 1984 Sep;99(3):894–899. doi: 10.1083/jcb.99.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]





