Abstract
Snake venom antagonists of α2β1 integrin have been identified as members of a C-lectin type family of proteins (CLP). In the present study, we characterized three new CLPs isolated from Echis sochureki venom, which interact with this integrin. These proteins were purified using a combination of gel filtration, ion exchange chromatography and reverse phase HPLC. Sochicetin-A and sochicetin-B potently inhibited adhesion of cells expressing α2β1 integrin and binding of isolated α2β1 ectodomain to collagen I, as well as bound to recombinant GST-α2A domain in ELISA, whereas activity of sochicetin-C in these assays was approximately two orders of magnitude lower. Structurally, sochicetin-B and sochicetin-C are typical heterodimeric αβ CLPs, whereas sochicetin-A exhibits a trimer of its subunits (αβ)3 in the quaternary structure. Immobilized sochicetins supported adhesion of glioma cell lines, LN18 and LBC3, whereas in a soluble form they partially inhibited adhesion of these cells to collagen I. Glioma cells spread very poorly on sochicetin-A, showing no cytoskeleton rearrangement typical for adhesion to collagen I or fibronectin. Adhesion on CLP does not involve focal adhesion elements, such as vinculin. Sochicetin-A also inhibited collagen-induced platelet aggregation, similar to other CLPs’ action on the blood coagulation system.
Keywords: C-lectin type proteins, collagen receptors, integrins, snake venom proteins, glioma cells, cell adhesion
Introduction
Snake venoms are a mixture of proteins, which exert a wide range of pharmacological activities. Although many of these proteins demonstrate enzymatic activity, snake venoms also contain non-enzymatic toxins inhibiting cell adhesion through interaction with the integrin receptors. Snake venom disintegrins are the most common antagonists of integrins. Different subclasses of disintegrins recognize RGD-dependent integrins such as αIIbβ3, αvβ3, α5β1; leukocyte integrins, α4β1 and α9β1, or collagen receptor, α1β1 integrin (for review: Marcinkiewicz, 2005; Swenson et al., 2007; McLane et al., 2008; Walsh and Marcinkiewicz, 2011). In addition to disintegrins, only one more family of snake venom proteins shows anti-integrin activity. Members of this family, C-lectin type proteins (abbreviated: CLPs, CTLs or Snaclecs), selectively antagonize the collagen receptor, α2β1 integrin. CLPs have been intensively investigated as modulators of the blood coagulation system. They interact with blood proteins such as factors IX/X and von Willebrand factor (Atoda et al., 1994; Maita et al., 2003), although most of them directly bind to receptors present on the platelet surface. Interestingly, some of CLPs work as inhibitors of platelet functions, whereas a large number of these proteins activate platelets (Clementson, 2010). GPIb, GPIV and α2β1 integrin are the major platelet receptors, which are affected by CLPs. Their antagonistic effect on α2β1 integrin is associated with blockage of collagen-induced platelet aggregation. No other integrins have been reported as a target for CLPs.
The majority of snake venom CLPs are heterodimers of two disulfide-bonded subunits, α and β. However, recent studies revealed that CLP may also adopt a heterotetrameric structure formed by four different subunits α, β, γ and δ. The prototypic CLP heterotetramer is rhodocetin, which specifically binds α2β1 integrin (Eble et al., 2009; Arlinghaus and Eble, 2012). Only three other CLPs have been reported as antagonists of this integrin, including EMS16 (Marcinkiewicz et al., 2000; Horii et al., 2003), VP12 (Staniszewska et al., 2009) and bilinexin (Du et al., 2001). The 3-D structure of EMS16 heterodimer has been modeled based on the crystal analysis of its molecular complex with A-domain of α2 integrin subunit (Horii et al., 2004). Additionally, structural analysis of other GPIb-binding CLPs such as botrocetin (Sen et al., 2001), bitiscetin (Maita et al., 2003), flavocetin-A (Fukuda et al., 2000), aggretin (Hooley et al., 2008) and convulxin (Batuwangala et al., 2004) has revealed higher oligomeric structures. For example flavocetin-A is a cyclic tetramer (αβ)4, whereas agglucetin subunits are arranged into double dimer (αβ)2 (Morita, 2005). Based on mass spectrometric analysis, a novel quaternary arrangement (αβ)3, has been proposed for a CLP found in Bitis nasicornis venom (Calvete et al., 2007). However, its structural analysis, and the functional characterization has not been reported. In this study, we present sochicetin-A, a novel α2β1 integrin-binding CLP, exhibiting an (αβ)3 structure, and two heterodimeric (αβ) CLPs, sochicetin-B and sochicetin-C. Sochicetin-A contains an extra cysteine, which appers to be crucial for forming cyclic oligomers (Morita, 2005).
Collagen receptor, α2β1 integrin is broadly expressed in the cells of various tissues (Santoro and Zutter 1995). It belongs to the subfamily of integrins containing A-domain (or I-domain) localized on the top of the N-terminal propeller domain of the α subunit (Dickeson and Santoro, 1998; Tulla et al., 2001). The A-domain harbors the collagen-binding site of α2β1 integrin (Emsley et al., 2000). Many reports characterized α2β1 integrin as a cell signaling molecule important in modulating cell physiological processes, such as proliferation and migration. It transfers cellular signals which are strongly linked to phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase p38 (MAPK p38) (Ivaska et al., 1999; Klekotka et al., 2001). This collagen receptor plays a role in cancer progression. Various cancer cells over-express this receptor on the cellular surface (Matsuoka et al., 2000; Mirtti et al., 2006), that also influences metastasis (Staniszewska et al., 2009; Hall et al., 2008; Ramirez et al., 2011). Moreover, it is present on the cancer-associated endothelial cells, and is important in the regulation of pathological angiogenesis (Senger et al., 1997; Zhang et al., 2008). In this study, we showed that α2β1 integrin expressed on glioma cell lines is specifically targeted by the new members of CLPs, which antagonize cell adhesion to collagen I.
Material and Methods
Antibodies, cell lines and other reagents
Snake venom of Echis sochureki was purchased from Latoxan Serpentarium (Valence, France). Monoclonal antibodies against α2β1 (clone P1E6) and α5β1 (clone SAM-1) integrins, as well as anti-vinculin (clone 7F9) and TRITC-labeled phalloidin were purchased from Millipore Inc. Polyclonal antibodies against α2 and α5 integrin subunits were purchased from Santa Cruz Biotech. Collagen type I from equine tendons and human plasma fibronectin was purchased from Chrono-Log Corp. and Millipore Inc, respectively. K562 cell line transfected with α2 integrin subunit (α2K562) was provided by Dr. M. Hemler (Dana Farber Cancer Institute, Boston, MA). Human erythroleukemic K562 and human glioma LN18 cell lines were purchased from ATCC. Human glioma LBC3 cell line was developed as described previously (Walsh et al., 2012).
Purification and structural characterization of sochicetins and their ethylpyridylated (EP)-subunits
Lyophilized venom was dissolved in 50 mM Tris-HCl, pH 7.0 (40 mg/0.6 ml) and separated on Superdex 200 column (2 × 100 cm) at a constant flow rate (2 ml/min). Collected fractions were concentrated and further purified on an ion-exchange chromatography and RP-HPLC. Fractionation on Mono-S column was performed in 50 mM Tris-HCl, pH 7.0 using the same flow conditions and elution with 0.8 M NaCl. RP-HPLC was performed using C18 column (25 × 1 cm) at a flow rate 2 ml/ml. First step of RP-HPLC was performed using linear acetonitrile gradient 0–80% in 0.1% TFA over 45 min. In the second step RP-HPLC time was increased to 120 min. Fractions collected were lyophilized after each step of RP-HPLC and reconstituted in water for further purification or for activity testing.
Separation of ethylpyridylated (EP)-subunits of sochicetins was performed according to a procedure described earlier (Marcinkiewicz et al., 2000; Bazan-Socha et la., 2004). Briefly, purified sochicetins (0.5 mg/ml) were dissolved in 0.1M Tris-HCL, pH 8.5 containing 4 mM EDTA and 6M guanidine hydrochloride, following reduction with 3.2 mM dithiothreitol (DTT). Reduced proteins were alkylated with 2-fold molar excess of 4-vinylpyridine over the reducing agent. EP-subunits of sochicetins were separated by RP-HPLC as described above. Isolated subunits were analyzed by N-terminal sequencing using an Applied Biosystems 477A instrument. Molecular masses of sochicetins and their subunits were evaluated by SDS-PAGE and confirmed by MALDI-TOF.
Cell adhesion studies
Adhesion studies of cultured cells labeled with 5-(chloromethyl)fluorescein diacetate (CMFDA) was performed as described previously (Marcinkiewicz et al., 2000). Briefly, CLPs, collagen I or fibronectin were immobilized on 96-well plate in PBS overnight at 4°C. The plate was blocked with 1% BSA in Hank’s balanced salt solution (HBSS) containing 5 mM MgCl2. Cells were labeled with 12.5 μM CMFDA and plated (1 × 105 per sample) into the wells in the presence or absence of sochicetins. Plates were incubated at 37°C for 60 min and unbound cells were removed by washing. Bound cells were lysed by 0.5% Triton X-100. The standard curve was prepared in parallel on the same plate using known concentration of labeled cells. Plate was read using fluorescence plate reader (Bio-Tek) with 485 excitation and 530 emission filters.
Titration of sochicetins with GST-tagged integrin α2 A-domain
Expression and purification of recombinant GST-tagged integrin α2 A-domain (GST-α2A) and ELISA experiments were performed as described previously (Eble et al., 2003). Briefly, sochicetins were immobilized at 20 μg/ml in TBS, pH 7.4 on 96-well plate overnight, following by blocking with 1% BSA. GST-α2A was added to the wells in TBS, supplemented with 2 mM MgCl2 and 1% BSA. Bound GST-α2A was detected with rabbit antibodies against GST (Invitrogen), diluted 1:1000, and with alkaline phosphatase-conjugated secondary antibodies. Substrate for alkaline phosphatase (para-nitro-phenyl-phosphate) was added, and plate was read using ELISA plate reader with 405 nm wavelength.
Inhibition of integrin α2β1 ectodomain binding to collagen I by sochicetins
The production of soluble integrin α2β1 ectodomain and its use in inhibition in ELISA have been described previously (Eble et al., 2001). Briefly, collagen I was coated at 40 μg/ml in 0.1M acetic acid. After blocking with 1% BSA, α2β1 integrin (18 μg/ml) was added to the wells in TBS, pH 7.4, supplemented with 1% BSA, 40 μg/ml integrin-activating mab (clone 9EG7) and 1 mM MnCl2. Different concentrations of sochicetins were added. Bound integrin was detected with rabbit polyclonal antibody against β1 integrin subunit and a secondary alkaline phosphatase-conjugated antibody with para-nitro-phenyl-phosphate as substratum.
Immunocytochemistry
Glass tissue culture chamber slides were coated with sochicetin-A, collagen I or fibronectin (all ligands with concentration 10 mg/ml) overnight at 4°C. Slides were blocked with 1% BSA in DMEM, then cells (1 × 106/ml) were added to the chambers and incubated for 2 hours at 37°C. Cells were fixed (20 min) with 4% paraformaldehyde and permeabilized for 5 minutes on ice with 0.2% Triton X-100 in PBS. Chambers were blocked by incubation with 10% horse or/and goat serum in 1% BSA for 1 hour at room temperature and primary antibody or antibodies (for double staining), were added. Incubation was continued for another 1–2 hours. After washing three times with PBS, appropriate FITC (green) or Texas Red (red) conjugated secondary antibodies were added and incubated for an additional 1 hour. Chambers were washed with PBS and coverslips were applied in mounting medium containing DAPI. Cells were observed under fluorescent microscope (Olympus IX81) with a 40x oil objective. MetaMorph digital imaging software was used for analysis.
Cell spreading analysis
Glass tissue chamber slides coating and cell adhesion procedures were performed as described for immunocytochemistry. Staining was completed by 1 hour incubation with TRITC-phalloidin following with mounting with buffer containing DAPI. Images of cells were captured using a fluorescent microscope (Olympus IX81) with a 40x oil objective. Calculation of spreading of a single cell area was performed using MetaMorph software for thresholded images. Statistical evaluation was based on calculation of at least 50 cells per group. Statistical analysis was performed using 1-way ANOVA with all pairwise multiple comparison procedures (Holm-Sidak method) for overall significance level = 0.05. Calculation was carried out using SigmaStat software (SPSS Inc., Chicago. IL).
Platelet aggregation
Platelet aggregation assay was performed using collagen I as an agonist according to the procedure described earlier (Marcinkiewicz et al, 2000).
Results
Fractionation of Echis sochureki venom
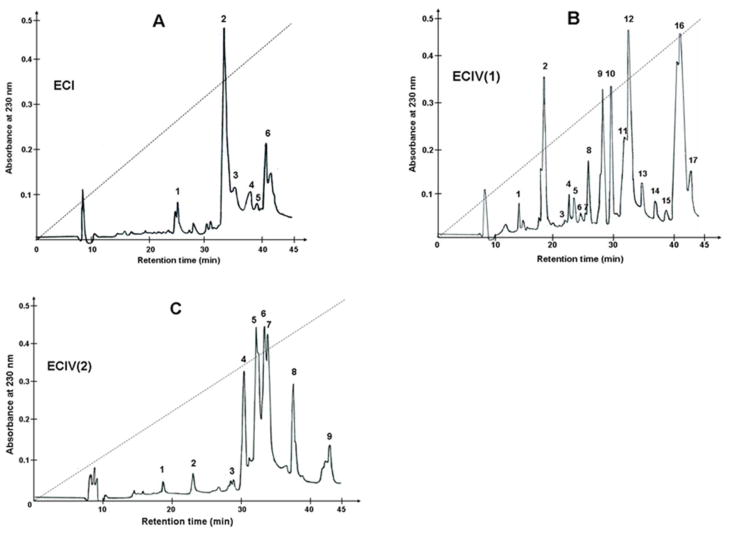
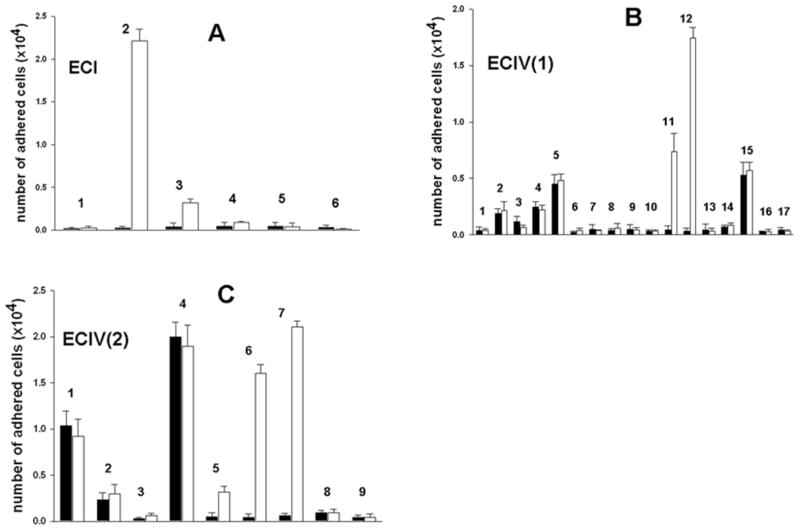
Purification of the α2β1 integrin inhibitors was accomplished using several chromatographic steps. Initial venom fractionation comprised gel filtration chromatography. Six major fractions were collected (Fig. 1). Pro-adhesive activity to α2β1 integrin was found in the first four fractions, termed ECI through ECIV (data not shown). ECI and ECIV exhibited the strongest activity were used for isolating α2β1 integrin antagonists. ECI was subfractionated by RP-HPLC, whereas ECIV was fractionated according to isoelectric points of proteins on cation-exchange column (Mono S) under neutral pH conditions. The flow-through fraction was named ECIV(1), and the subset of proteins eluted with 0.8 M NaCl comprised fraction ECIV(2). Reverse-phase chromatography of ECI on a C18 column resulted in 6 peaks (Fig. 2A). ECIV(1) and ECIV(2) were also applied on the RP-HPLC C18 column, yielding 17 and 9 peaks, respectively (Figs. 2B and 2C). ECI and ECIV RP-HPLC fractions were screened for selective adhesion to α2β1 integrin expressing K562 cells (Fig. 3). Proteins specifically interacting with α2β1 integrin were located in peaks eluting from C18 column between 31 and 34 minutes. The highest α2K562 binding-activity was associated with peaks ECI2, ECIV(1)12 and ECIV(2)7. These fractions did not show binding activity of control non-transfected K562 cells, which express only endogenous α5β1 integrin. Interestingly, several chromatographic peaks contained proteins that adhered to K562 cells. These peaks may include α5β1 integrin-binding components, i.e. monomeric or dimerc disintegrins. Thus, N-terminal sequence analysis revealed the major protein of ECIV(1)2 fraction is the monomeric short disintegrin, echistatin. The adhesion activity of α2K562 cells to immobilized EC fractions was correlated with their capability to inhibit cell adhesion to immobilized collagen type I (data not shown). ECI2, ECIV(1)12 and ECIV(2)7 were subjected for further structural and functional characterization. To this end, ECIV(1)12 and ECIV(2)7 were re-chromatographed on C18 column using a shallower acetonitrile gradient. Purified proteins expressing anti-α2β1 integrin activity were named sochicetins: ECI2, sochicetin-A; ECIV(1)12, sochicetin-B; and ECIV(2)7 sochicetin-C.
Fig. 1.
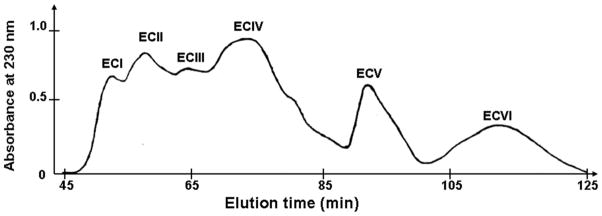
Gel filtration chromatographic separation of Echis sochureki venom. 40 mg of crude venom in 1 ml were applied on a Superdex 200 column column equilibrated with 50 mM Tris-HCl, pH 7.0. Elution was performed at a flow rate of 2 ml/min.
Fig. 2.
RP-HPLC profile of EC fractions. (A) ECI; (B) ECIV(1); (C) ECIV(2) fractions (5 mg in 0.5 ml) were applied on C18 column and elution was performed with linear gradient (dashed lines) of increased concentration of acetonitrile (0–80%) in 0.1% TFA. Fractions were collected manually and lyophilized.
Fig. 3.
Screening of fractions ECI (A), ECIV(1) (B), and ECIV(2) (C) for pro-adhesive properties in the cell adhesion assay. Fractions (1 μg in 0.1 ml of PBS) were immobilized on 96-well plate by overnight incubation at 4°C. K562 cells (filled bars) and α2K562 cells (open bars) were labeled with CMFDA and applied on the wells previously blocked with 1%BSA. After 30 min incubation, unbound cells were removed by washing, whereas adhered cells were lysed with 0.5% Triton X-100. Plate was read with fluorescence plate reader using 485 nm excitation and 530 nm emission filters. Error bars represent S.D. from three independent experiments.
Structural characterization of sochicetins
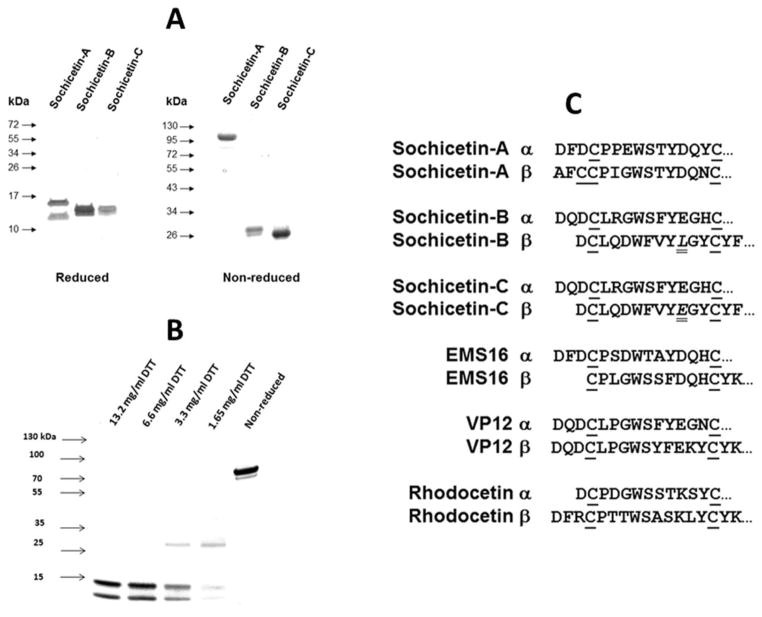
Structural analysis of purified proteins was performed by SDS-PAGE and N-terminal sequencing of ethylpyridylated (EP) subunits of sochicetins (Fig. 4). SDS-PAGE revealed that sochicetin-B and sochicetin-C appear to be similar proteins (Fig. 4A). In non-reduced gel they migrate as single bands with apparent molecular weight around 30 kDa. After reduction, both proteins show two bands of approximately 15 kDa. This data indicated that sochicetin-B and sochicetin-C are canonical heterodimers, and this hypothesis was verified by mass spectrometry showing molecular masses for sochicetin-B and -C of 30,720 and 30,742 Da, respectively. On the other hand, sochicetin-A is structurally distinct (Fig. 4A). Under non-reducing conditions, sochicetin-A ran as a 90 kDa band, whereas upon reduction yielded two bands of 17 and 14 kDa. These results suggested that sochicetin-A exhibits a multimeric structure, which according to the molecular weights of its reduced subunits appears to be a hexamer. Mass spectrometric analysis yielded an isotope-averaged molecular mass of 90,124 Da, thus confirming the SDS-PAGE estimation. The analysis of a partial reduction of sochicetin-A on SDS-PAGE revealed dissociation of hexamer to the heterodimer (molecular weight approximately 30 kDa) under 1.65 mg/ml concentration of DTT used as a reducing agent (Fig. 4B). Complete reduction of the molecule to single subunits occurred under 6.6 mg/ml DTT.
Fig. 4.
Structural characterization of sochicetin-A, -B and -C. (A) SDS-PAGE of reduced and non-reduced samples. Proteins (10 μg) were loaded on the 10% and 15% gels for reducing and non-reducing conditions, respectively. Protein bands were visualized by Coomassie blue staining. Molecular weight markers are indicated by arrows. (B) SDS-PAGE of sochicetin-A in different stage of reduction. Proteins (10 μg) were loaded on the gradient gel (4–20%) in the absence or presence of different concentrations of DTT. Protein bands were visualized as above. (C) EP-subunits of proteins were separated on RP-HPLC and N-terminal sequences of amino acids were determined. N-terminal amino acid sequences of sochicetins are compared with other CLPs inhibiting α2β1 integrin. Cysteine residues are aligned and underlined. Difference in amino acid composition between sochicetin-B and sochicetin-C is in italic and double underline.
N-terminal sequencing of first 15 amino acids of separated EP-subunits confirmed our expectations that sochicetin-A, -B, and -C belong to the CLP family of proteins (Fig. 4C). Sochicetin-B and sochicetin-C differ only by one amino acid in subunit β (10Leu→10Glu), and exhibit high similarity to other CLPs, which selectively interact with α2β1 integrin, such as EMS16 (Hori et al, 2003), VP12 (Staniszewska et al, 2009) and rhodocetin (Wang et al, 1999) (Fig. 4C). On the other hand, the N-terminal amino acid sequence of sochicetin-A subunit β departs from that of other homologous CLP subunits, as it contains an extra cysteine, possibly involved in the multimerization of αβ dimers (Calvete et al., 2007; Arlinghaus and Eble, 2012). Extra cysteine residues at both, the C-terminus of the α-chain and the N-terminus of the βsubunit, enabling multimerization of αβ-heterodimers via inter-dimer disulfide linkages into a cyclic “head-to-tail” (αβ)n arrangement. This class of multimeric CLPs has not been reported as antagonists of the α2β1 integrin
Functional characterization of CLPs isolated from Echis sochureki venom
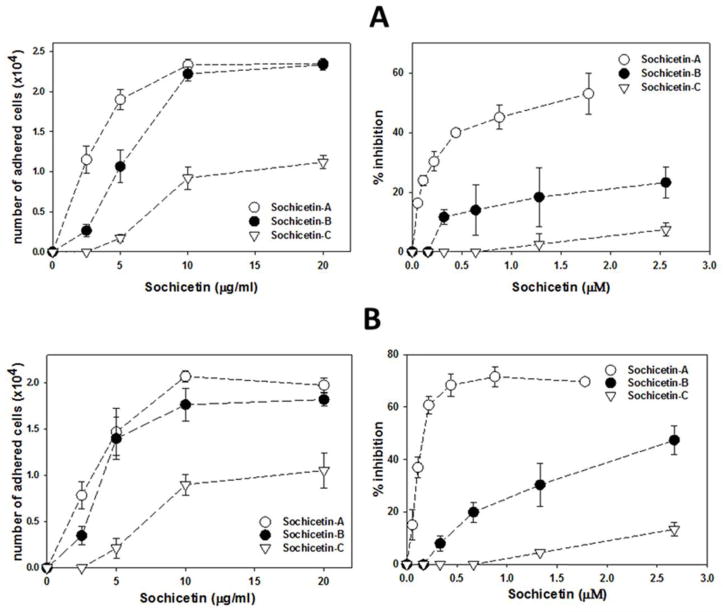
Direct interaction of CLPs isolated from Echis sochureki venom with α2β1 integrin was investigated in cell adhesion and ELISA assays. All three sochicetin proteins showed inhibitory effect on binding of α2β1 integrin to collagen I, although the potency of this inhibition was different (Fig. 5). The most potent blocker of the adhesion of α2K562 cells to collagen I was sochicetin-A (IC50 = 1.38 nM), whereas the inhibitory effect of sochicetin-C was approximately two orders of magnitude lower (IC50 = 265.1 nM) (Fig. 5A). Sochicetin-B inhibited α2β1 integrin with less potency than that of sochicetin-A but much stronger than sochicetin-C, with an IC50 of 7.3 nM. The possible antagonistic effect of sochicetins for other integrins was also assessed using specific cell adhesion assays. In concentrations of up to 10 μM, none of the sochicetins showed inhibitory effect on the collagen receptor, α1β1 integrin, or β1 integrins such as α4, α5 and α9 (data not shown). The ability of the sochicetins to block soluble α2β1 integrin ectodomain to immobilized collagen I was tested in ELISA (Fig. 5B). In this assay, sochicetin-B (IC50 = 32.7 nM) and sochicetin-A (IC50 = 59.7 nM) displayed similar potency, while sochicetin-C showed a much lower inhibitory effect (IC50 = 1478 nM). This data pointed to the A-domain of α2 subunit as responsible for the integrin’s ligand specificity and potency. To check this assumption, we analyzed the direct binding of recombinant GST-tagged integrin α2 A-domain (GST-α2A) to immobilized sochicetin proteins in ELISA (Fig. 5C). GST-α2A bound to sochicetin-A and sochicetin-B with high affinity showing Kd values of 11.72 nM and 17.30 nM, respectively. Only a weak interaction was observed with sochicetin-C (Kd = 54.5 nM).
Fig. 5.
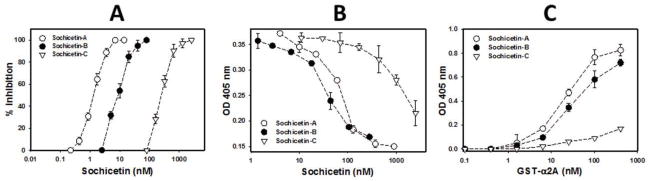
Effect of purified CLPs on interaction of α2β1 integrin with collagen I. (A) Cell adhesion assay was performed as described in Fig. 3 legend using α2K562 cells. Collagen I (10 μg/ml) was immobilized on 96-well plate by overnight incubation at 4°C. Cells were mixed with CLPs at indicated concentrations and pre-incubated at room temperature by 15 min before application on the plate. (B) Inhibition of α2β1integrin binding to immobilized collagen I. Sochicetins were mixed with soluble α2β1 integrin ectodomain (18 μg/ml) and applied on the plate. Collagen-I-bound α2β1 integrin was quantified by ELISA. (C) Direct interaction of sochicetins with recombinant GST-α2A in ELISA. Sochicetins (20 μg/ml) were immobilized on 96-well plate by overnight incubation in TBS, pH 7.4 at 4°C. Plate was blocked with BSA and GST-α2A was incubated on the plate for 1 hour. Detection of bound GST-α2A was performed using polyclonal anti-GST antibody following alkaline phosphatase conjugated anti-rabbit IgG secondary antibody. Plate was read with ELISA plate reader using 405 nm wavelength. Means and SD are shown from experiments performed in duplicates and repeated at least three times
The activity of sochicetin-A on collagen I-induced platelet aggregation was also tested. Platelets isolated from human blood were used for this experiment. Sochicetin-A potently inhibited platelet aggregation with an IC50 of 20 nM, similar to the activity reported for other α2β1 interacting CLPs, such as EMS16 (Marcinkiewicz et al, 2000).
Interaction of sochicetins with glioma cells
The effect of sochicetins on glioma cell lines, which endogenously express α2β1 integrin, was investigated (Fig. 6). LN18 and LBC3 cell lines express high level of collagen receptors, including α1β1 and α2β1 integrins. LN18 cells (Fig. 6A), as well as LBC3 cells (Fig. 6B) adhered to sochicetin-A and sochicetin-B with similar potency and reached saturation approximately at 10 μg/ml of immobilized protein. These cells adhered very poorly to sochicetin-C, with much lower number of cells adhering to sochicetin-A and -B saturating conditions. None of the CLPs isolated from Echis sochureki venom completely inhibited glioma cell lines adhesion to collagen I (Fig. 6, right panels). However, the blocking effect of sochicetin-A was also the most potent, achieving 50 and 70% inhibition for LN18 and LBC3 cells, respectively. Very little activity was observed for sochicetin-C, whereas sochicetin-B showed an intermediate effect.
Fig. 6.
Interaction of glioblastoma cell lines with CLPs isolated from Echis sochureki venom. LN18 cells (A) or LBC3 (B) cells were tested for adhesion to immobilized CLPs (left panels) or for inhibition of adhesion to immobilized collagen I by CLPs. CLPs at different concentrations or collagen I (10 μg/ml) were immobilized on 96-well plate. LN18 and LBC3 cells were labeled with CMFDA and cell adhesion experiment was performed as described in Fig. 3 legend. The error bars represent S.D. from three independent experiments.
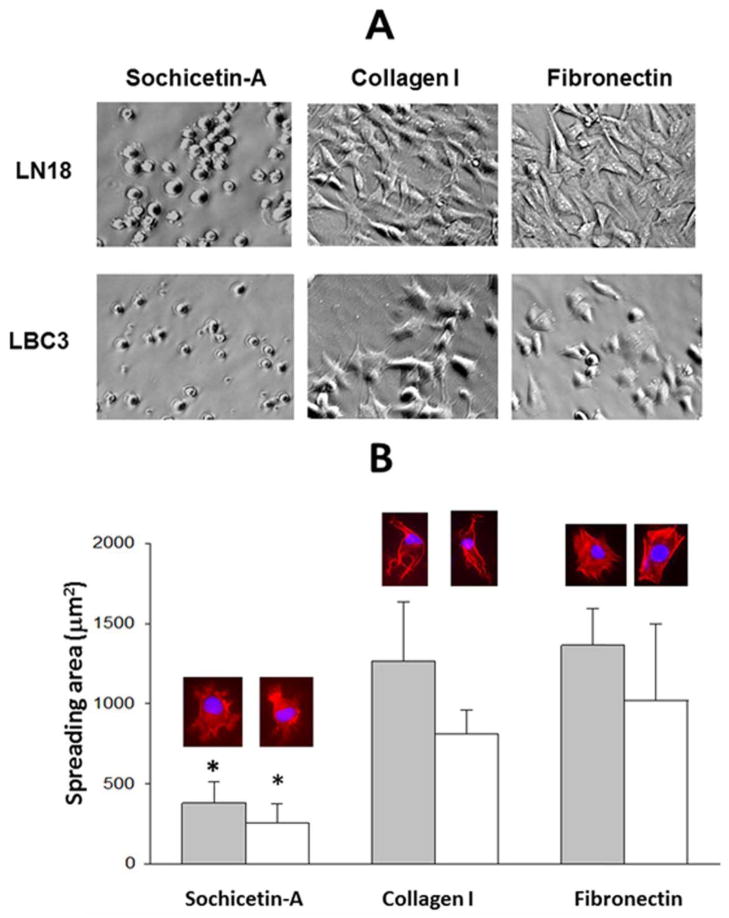
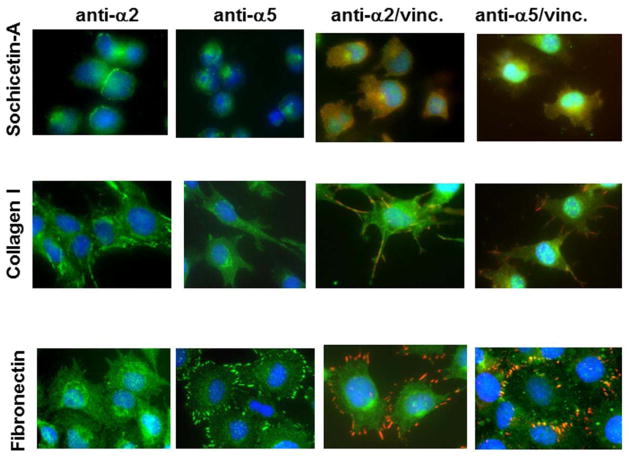
Sochicetin-A and -B bind to α2β1 integrin with high affinity. Therefore, we assessed whether immobilized sochicetin would support adhesion and spreading to a similar extent as collagen I (Fig. 7). Fibronectin was used as a control ECM protein in this assay. Spreading of glioma cell lines was very poor on the sochicetin-A. After one hour incubation, round-shaped cells with a small contact area to the sochicetin-A-coated surface were observed (Fig. 7A). On the contrary, cells were fully spread on the ECM proteins, collagen I and fibronectin. Staining of cell cytoskeleton with phalloidin confirmed low cell spreading area for both glioma cell lines on sochicetin-A, which was statistically significantly higher when collagen I and fibronectin were used as substrates (Fig. 7B). Differences between the morphology of cells seeded on specific ligands were also observed. An atypical actin organization with no stress fibers formation was observed in cells on sochicetin-A, although numerous filopodia were locally developed (Fig. 7B). Cells spread on collagen were elongated with a fibroblast-like appearance, whereas on fibronectin cells were fully spread and showed well organized, actin stress fibers. Association of α2β1 integrin with these fibril-like structures during attachment of LN18 cells to collagen I was confirmed by immunocytochemistry (Fig. 8). On the other hand, cells seeded on fibronectin showed no focal contacts, as assessed by immunostaining with anti-α2 mab. Similar observation was performed for sochicetin-A. Although phalloidin staining (Fig. 7B) indicated poor cell spreading, an increased presence of α2β1 integrin was shown on the cell periphery, especially at cell-cell contacts (Fig. 8). Cells seeded onto sochicetin-A and collagen I showed an even distribution of anti-α5 mab staining, indicating that α5β1 integrin was not recruited into focal adhesion. However, cell cultured on fibronectin intensively gather α5β1 integrin into focal adhesion as demonstrated by staining for anti-α5 in cell areas that also stained for vinculin, a marker for focal adhesion. Co-localization of α2β1 integrin with vinculin was observed when cells were seeded on collagen I, but not on sochicetin-A (Fig. 8).
Fig. 7.
Spreading of LN18 and LBC3 cells on sochicetin-A, collagen I and fibronectin. (A) Proteins (10 μg/ml) were immobilized on 6-well plate by overnight incubation at 4°C. Cells (2 × 105/ml) were added to the wells and incubated for 1 hour. Unattached cells were washed off and cells were fixed by 2% paraformaldehyde. Cells were analyzed under phase-contrast microscope with 150x magnification. (B) Evaluation of LN18 (gray bars) and LBC3 (open bars) cell spreading area was performed on glass chamber slides, with immobilized specific ligand as described above. Adhered cells were stained with phalloidin and mounted with buffer containing DAPI. Representative images of spreaded cells are above the bars. Error bars represent S.D. calculated from analysis at least 50 cells per group. (*) indicate statistically significant difference between collagen I and fibronectin group (p<0.001).
Fig. 8.
Immunocytostaining of LN18 cells plated onto sochicetin-A, collagen I and fibronectin. Cells were seeded on glass slides with previously immobilized protein. After blocking, primary mabs against α2 or α5 integrin subunit were applied. FITC-labeled horse anti-mouse IgG (green) was used for specify fluorostaining. Double immunostaining was performed using polyclonal anti-α2 and anti-α5 (green) and monoclonal anti-vinculin (red) antibodies, following application of mixture of FITC-anti-rabbit IgG and Texas Red-anti-mouse IgG. Mounting buffer containing DAPI was added in the final step for visualization of nucleus (blue). Cells were analyzed (400x magnification) using Olympus IX81 fluorescent microscope.
Discussion
CLPs represent a steadily growing family of snake venom molecules exhibiting diverse biological activities. They were isolated from the venom of several of viper species, and showed diversity in biological activities. Four CLPs targeting collagen receptor, integrin α2β1, have been identified to date. Here we report three novel α2β1 integrin-inhibiting CLPs, which are present in one venom of the Eastern saw-scaled viper Echis sochureki. Two of them, sochicetin-B and sochicetin-C, display the typical the canonical αβ hereodimeric structure of the majority of other CLPs. On the other hand, sochicetin-A contains unique structural feature. It consists of two subunits, which are associated by disulfide bonds in a hexameric structure. Organization of this hexamer is structurally not established by protein crystallography yet, but based on the data presented for other oligomeric CLPs (Morita, 2005), we propose that molecule of sochicetin-A is formed as trimeric aggregate of heterodimers, and has a quaternary (αβ)3 structure. Such structure of sochicetin-A appears to be supported by the presence of an extra cysteine in N-terminus of its subunit β. Flavocetin-A, convulxin and mucrocetin also contain cysteine in the same N-terminal position as sochicetin-A, which is responsible for a “head-to-tail” arrangement of cyclic structure (αβ)4 by creating a disulfide bridge with an extra cysteine present on the C-terminus of a subunit α (Fukuda et al., 2000; Batuwangala et al., 2004; Huang et al., 2004). Experiment with partial reduction of sochicetin-A supports this concept (Fig. 4B). This molecule is reduced to the heterodimer by a lower concentration of DTT, and further to the single subunits by higher concentration of DTT. It suggests that basic heterodimers are associated to the trimeric form, which according to the cyclic models of other oligomeric CLPs are connected by single, more sensitive for DTT disulfide bond.
Sochicetin-C has very weak binding affinity to α2β1 integrin. It co-purifies with echicetin, a GPIb-binding CLP, which structurally is also in heterodimeric form (Peng et al., 1993; Navdaev et al., 2001). The similarity in protein properties between sochicetin-C and echicetin suggest that these CLPs may have relationships in targeting specific receptors. Although we excluded echicetin as α2β1 integrin-binding component of Echis sochureki venom, sochicetin-C may interact with GPIb. Testing this CLP in GPIb activity systems will verify this hypothesis, which is based on the activity of other multifunctional CLPs such as bilinexin (Du et al., 2001). Sochicetin-B is similar to the EMS16, a CLP isolated from the venom of evolutionary close species, Echis multisquamatus (Marcinkiewicz et al., 2000), although its α2β1 integrin inhibitory potency in a cell adhesion assay, (7.3 nM) is over 10-fold lower than that of EMS16 (0.5 nM). On the other hand, in the same assay another heterodimeric CLP, VP12 isolated from Vipera palestinae venom has intermediate activity (IC50 = 3.6 nM) (Staniszewska et al., 2009).
In all cell adhesion experiments sochicetin-A was more potent, regardless of its use as an immobilized ligand or soluble inhibitor of cell adhesion to immobilized natural ligand, collagen I. However, a different situation occurs when sochicetin-A and sochicetin-B were tested in ELISA for the ability to bind the recombinant A-domain of α2 integrin subunit. Immobilized sochicetin-A exhibited stronger binding activity towards soluble A-domain, whereas sochicetin-B inhibited more efficiently the binding of soluble form of α2β1 integrin ectodomain to immobilized collagen I. These results indicate that multimerization may have an impact on CLP-α2β1 active complex formation. The structural basis of the interaction between heterodimeric EMS16 and α2A-domain were analyzed by X-ray crystallography, and it involves the distal regions of the concave surface of this CLP molecule (Horii et al., 2004). The cyclic “head-to-tail” (αβ)3 arrangement may create new α2β1-interacting regions that explain why hexameric sochicetin-A more efficiently antagonizes the adhesive function of α2β1 integrin expressed on the cellular membrane, than dimeric sochicetin-B. Whether this effect is due to multimeric CLPs binding with stronger affinity to integrin in resting stage than dimeric CLPs, as shown recently for tetrameric rhodocein (Arlinghaus and Eble, 2012; Bracht et al., 2011), deserves further studies. In addition integrin’s conformation thereby enhancing the affinity of the ligand/receptor interaction remains also elusive.
Sochicetins are novel biochemical tools to investigate structure-function correlations of the α2β1 integrin in cell physiology and particularly in tumor biology. Although the role of α2β1 integrin was examined in various pathologies including breast cancer (Ramirez et al., 2011), its significance for glioma progression has never been reported. The presence of this integrin on glioma cells was demonstrated by immunohistochemistry (Paulus et al., 1993), and we also found its high expression on glioma cell lines, LN18 and LBC3. These cells strongly interact with collagen I and this interaction was antagonized by sochicetins. However, none of the investigated CLPs were able to completely abolish the adhesion of glioma cells to collagen I, as was the case for α2K562 cell. This suggests that glioma cells may express collagen receptors other than α2β1 integrin. Previously, we found that simultaneous blocking of α1β1 integrin increased the ability of VP12 to completely abolish adhesion of melanoma cell lines to collagen I (Staniszewska et al., 2009). A similar situation may occur for glioma cell lines, which also express another collagen receptor, α1β1 integrin (data not shown). Separate studies with blockers of these two collagen receptors are required to proof this concept.
Although the two glioma cell lines investigated adhered to immobilized sochicetins, a significant difference was observed for their spreading when compared with endogenous ECM proteins. Sochicetin-A fails to induce integrin clustering into focal adhesion formation, and consequently, actin stress fibers formation, resulting in roundish cell shape. In this context, comparison of CLP with collagen I suggests ligand diversity to induce cell signaling. Therefore, we consider sochicetin-A as an antagonist of α2β1 integrin-related cell functions. Further studies of structural relation of CLPs with their function may result in designing new pharmaceuticals antagonizing pathological outcomes of α2β1 integrin.
Highlights.
Isolation of three novel snake venom CLPs inhibiting α2β1 integrin
Reporting hexameric CLP, sochicetin-A, with anti-collagen receptor activity
CLPs antagonize the interaction of glioma cells with collagen matrix
Sochicetin-A does not support glioma cell spreading
Acknowledgments
This work was supported in part by NIH NCI (grant 1R01CA133262 to C.M.); German-Israeli foundation (grant No. 994-3.9/2008 to J.A.E. and P.L.); David R. Bloom Center for Pharmacy and the Dr. Adolf and Klara Brettler Center for Research in Molecular Pharmacology and Therapeutics at The Hebrew University of Jerusalem, Israel (to P.L.); grant BFU2010-17373 from the Ministerio de Economía y Competitividad, Madrid, Spain (to J.J.C).
Abbreviations
- CLP
C-lectin type protein
- CMFDA
5-(cloromethyl)fluorescein diacetate DTT dithiothreitol
- ECM
extracellular matrix
- EP
ethylpyridylated
- GST-α2A
GST-tagged integrin α2 A-domain
- RP-HPLC
reverse phase – high performance liquid chromatography
- TFA
trifluoroacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arlinghaus FT, Eble JA. C-type lectin-like proteins from snake venoms. Toxocon. 2012;60:512–519. doi: 10.1016/j.toxicon.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Atoda H, Yoshida N, Ishikawa M, Morita T. Binding properties of the coagulation factor IX/factor X-binding protein isolated from the venom of Trimerusurus flavoviridis. Eur J Biochem. 1994;224:703–708. doi: 10.1111/j.1432-1033.1994.t01-1-00703.x. [DOI] [PubMed] [Google Scholar]
- Batuwangala T, Leduc M, Gibbins JM, Bon C, Jones EY. Structure of the snake- venom toxin convulxin. Acta Crystallogr D Biol Crystallogr. 2004;60:46–53. doi: 10.1107/s0907444903021620. [DOI] [PubMed] [Google Scholar]
- Bazan-Socha S, Kisiel DG, Young B, Theakston RRDG, Calvete JJ, Sheppard D, Marcinkiewicz C. Structural requirements of MLD-containing disintegrins for functional interaction with α4β1 and α9β1 integrins. Biochemistry. 2004;43:1639–1647. doi: 10.1021/bi035853t. [DOI] [PubMed] [Google Scholar]
- Bracht T, Figueiredo de Rezende F, Stetefeld J, Sorokin LM, Eble JA. Monoclonal antibodies reveal the alteration of the rhodocetin structure upon α2β1 integrin binding. Biochem J. 2011;440:1–11. doi: 10.1042/BJ20110584. [DOI] [PubMed] [Google Scholar]
- Calvete JJ, Escolano J, Sanz L. Snake venomics of Bitis species reveals large intragenus venom toxin composition variation: application to taxonomy of congeneric taxa. J Proteome Res. 2007;6:2732–2745. doi: 10.1021/pr0701714. [DOI] [PubMed] [Google Scholar]
- Clementson KJ. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon. 2010;56:1236–1246. doi: 10.1016/j.toxicon.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Dickeson SK, Santoro SA. Ligand recognition by the I domain-containing integrins. Cell Mol Life Sci. 1998;54:556–566. doi: 10.1007/s000180050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XY, Navdaev A, Clemetson JM, Magnenat E, Wells TN, Clemetson KJ. Bilinexin, a snake C-type lectin from Agkistrodon bilineatus venom agglutinates platelets via GPIb and α2β1. Thromb Haemost. 2001;86:1277–1283. [PubMed] [Google Scholar]
- Eble J, Beermann B, Hinz HJ, Schmidt-Hederich A. Alpha2beta1 integrin is not recognized by rhodocytin but is the specific, high affinity target of rhodocetin, an RGD- independent disintegrin and potent inhibitor of cell adhesion to collagen. J Biol Chem. 2001;276:12274–12284. doi: 10.1074/jbc.M009338200. [DOI] [PubMed] [Google Scholar]
- Eble JA, Tuckwell DS. The alpha2beta1 integrin inhibitor rhodocetin binds to the A- domain of the integrin alpha2 subunit proximal to the collagen-binding site. Biochem J. 2003;376:77–85. doi: 10.1042/BJ20030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble JA, Niland S, Bracht T, Mormann M, Katalinic JP, Pohlentz P, Stetefeld J. The α2β1 integrin-specific antagonist rhodocetin is a cruciform, heterotetrameric molecule. FASEB J. 2009;23:2917–2927. doi: 10.1096/fj.08-126763. [DOI] [PubMed] [Google Scholar]
- Emsley J, Knight CG, Farndale RW, Barnes MJ, Liddinton RC. Structural basis of collagen recognition by integrin alpha2beta1. Cell. 2000;101:47–56. doi: 10.1016/S0092-8674(00)80622-4. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Mizuno H, Atoda H, Morita T. Crystal structure of flavocetin-A, a platelet glycoprotein Ib-binding protein, reveals a novel cyclic tetramer of C-type lectin-like heterodimers. Biochemistry. 2000;39:1915–1923. doi: 10.1021/bi992134z. [DOI] [PubMed] [Google Scholar]
- Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley E, Papagrigoriou E, Navdaev A, Pandey AV, Clemetson JM, Clemetson KJ, Emsley J. The crystal structure of the platelet activator aggretin reveals a novel (αβ)2 dimeric structure. Biochemistry. 2008;47:7831–7837. doi: 10.1021/bi800528t. [DOI] [PubMed] [Google Scholar]
- Horii K, Okuda D, Morita T, Mizuno H. Structural characterization of EMS16, an antagonist of collagen receptor (GPIa/IIa) from the venom of Echis multisquamatus. Biochemistry. 2003;42:12497–12502. doi: 10.1021/bi034890h. [DOI] [PubMed] [Google Scholar]
- Horii K, Okuda D, Morita T, Mizuno H. Crystal structure of EMS16 in complex with the integrin α2-I domain. J Mol Biol. 2004;341:519–527. doi: 10.1016/j.jmb.2004.06.036. [DOI] [PubMed] [Google Scholar]
- Huang KF, Ko TP, Hung CC, Chu J, Wang AH, Chiou SH. Crystal structure of a platelet-agglutinating factor isolated from the venom of Taiwan habu (Trimeresurus mucrosquamatus) Biochem J. 2004;378:399–407. doi: 10.1042/BJ20031507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kähäri VM, Heino J. Integrin α2β1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the α2 cytoplasmic tail. J Cell Biol. 1999;147:401–416. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klekotka PA, Santoro SA, Zutter MM. alpha 2 integrin subunit cytoplasmic domain- dependent cellular migration requires p38 MAPK. J Biol Chem. 2001;276:9503–9511. doi: 10.1074/jbc.M006286200. [DOI] [PubMed] [Google Scholar]
- Maita N, Nishio K, Nishimoto E, Matsui T, Shikamoto Y, Morita T, Sadler JE, Mizuno H. Crystal structure of von Willebrand factor A1 domain complexed with snake venom bitiscetin: insights into glycoprotein Ibα binding mechanism induced by snake venom proteins. J Biol Chem. 2003;279:37777–37781. doi: 10.1074/jbc.M305566200. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C, Lobb RR, Marcinkiewicz MM, Daniel JL, Smith JB, Dangelmaier C, Weinreb PH, Beacham DA, Niewiarowski S. Isolation and characterization of EMS16, a C-lectin type protein from Echis multisquamatus venom, a potent and selective inhibitor of the α2β1 integrin. Biochemistry. 2000;39:9859–9867. doi: 10.1021/bi000428a. [DOI] [PubMed] [Google Scholar]
- Marcinkiewicz C. Functional Characteristic of snake venom disintegrins: Potential therapeutic implication. Curr Pharm Des. 2005;11:815–827. doi: 10.2174/1381612053381765. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Yashiro M, Nishimura S, Inoue T, Fujihara T, Sawada T, Kato Y, Seki S, Hirakawa Y, Chung K. Increased expression of alpha2beta1-integrin in the peritoneal dissemination of human gastric carcinoma. Int J Mol Med. 2000;5:21–25. doi: 10.3892/ijmm.5.1.21. [DOI] [PubMed] [Google Scholar]
- McLane MA, Joerger T, Mahmoud A. Disintegrins in health and disease. Front Biosci. 2008;13:6617–6637. doi: 10.2741/3177. [DOI] [PubMed] [Google Scholar]
- Mirtti T, Nylund C, Lehtonen L, Hiekkanen H, Nissinen L, Kallajoki M, Alanen K, Gullberg D, Heino J. Regulation of prostate cell collagen receptors by malignant transformation. Int J Cancer. 2006;118:889–898. doi: 10.1002/ijc.21430. [DOI] [PubMed] [Google Scholar]
- Morita T. Structures and functions of snake venom CLPs (C-type lectin-like proteins) with anticoagulant-, procoagulant-, and platelet-modulating activities. Toxicon. 2005;45:1099– 1114. doi: 10.1016/j.toxicon.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Navdaev A, Dormann D, Clemetson JM, Clemetson KJ. Echicetin, a GPIb-binding snake C-type lectin from Echis carinatus, also contains a binding site for IgMk responsible for platelet agglutination in plasma and inducing signal transduction. Blood. 2001;97:2333–2341. doi: 10.1182/blood.v97.8.2333. [DOI] [PubMed] [Google Scholar]
- Paulus W, Baur I, Schauppan D, Roggendorf W. Characterization of integrin receptors in normal and neoplastic human brain. Am J Pathol. 1993;143:154–163. [PMC free article] [PubMed] [Google Scholar]
- Peng M, Lu W, Beviglia L, Niewiarowski S, Kirby EP. Echicetin: a snake venom protein that inhibits binding of von Willebrand factor and alboaggregins to platelet glycoprotein Ib. Blood. 1993;81:2321–2328. [PubMed] [Google Scholar]
- Ramirez NE, Zhang Z, Madamanchi A, Boyd KL, O’Rear LD, Nashabi A, Li Z, Dupont WD, Zijlstra A, Zutter MM. The α2β1 integrin is a metastasis suppressor in mouse models and human cancer. J Clin Invest. 2011;121:226–237. doi: 10.1172/JCI42328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro SA, Zutter MM. The alpha 2 beta 1 integrin: a collagen receptor on platelets and other cells. Thromb Haemost. 1995;74:813–821. [PubMed] [Google Scholar]
- Sen U, Vasudevan S, Subbarao G, McClintock RA, Celikel R, Ruggeri ZM, Varughese KI. Crystal structure of the von Willebrand factor modulator botrocetin. Biochemistry. 2001;40:345–352. doi: 10.1021/bi0021737. [DOI] [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci USA. 1997;94:13612–13617. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staniszewska I, Walsh EM, Rothman VL, Lazarovici P, Calvete JJ, Tuszynski GP, Gaathon A, Marcinkiewicz C. Effect of VP12 and viperistatin on collagen receptors-dependent promotion of melanoma metastasis. Cancer Biol Therap. 2009;8:55–64. doi: 10.4161/cbt.8.15.8999. [DOI] [PubMed] [Google Scholar]
- Swenson S, Ramu S, Markland RS. Anti-angiogenesis and RGD-containing snake venom disintegrins. Curr Pharm Des. 2007;13:2860–2871. doi: 10.2174/138161207782023793. [DOI] [PubMed] [Google Scholar]
- Tulla M, Pentikäinen OT, Viitasalo T, Käpylä J, Impola U, Nykvist P, Nissinen L, Johnson MS, Heino J. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem. 2001;276:48206–48212. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- Walsh EM, Marcinkiewicz C. Non-RGD-containing snake venom disintegrins, functional and structural relations. Toxicon. 2011;58:355–362. doi: 10.1016/j.toxicon.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Walsh EM, Kim R, Weaver M, Del Valle L, Sheffield J, Lazarovici P, Marcinkiewicz C. Importance of interaction of NGF with α9β1 integrin in progression of glioma angiogenesis. Neuro-Oncol. 2012;14:890–901. doi: 10.1093/neuonc/nos119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Kini RM, Chung MC. Rhodocetin, a novel platelet aggregation inhibitor from the venom of Calloselasma rhodostoma (Malayan pit viper): synergistic and noncovalent interaction between its subunits. Biochemistry. 1999;38:7584–7593. doi: 10.1021/bi982132z. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Ramirez NE, Yankeelov TE, Li Z, Ford LE, Qi Y, Pozzi A, Zutter MM. α2β1 integrin expression in the tumor microenvironment enhances tumor angiogenesis in a tumor cell specific manner. Blood. 2008;11:1980–1988. doi: 10.1182/blood-2007-06-094680. [DOI] [PMC free article] [PubMed] [Google Scholar]