Abstract
The arrival of ipilimumab and vemurafenib has provided oncologists with new choices in a field in which options were slim and the prognosis was grim. Combination therapy may lead to an improved survival benefit.
Introduction
The year 2011 brought two important advances in the treatment of metastatic melanoma, thanks to the FDA’s approval of novel therapies aimed at specific molecular targets in this disease—ipilimumab (Yervoy, Bristol-Myers Squibb)1 and vemurafenib (Zelboraf, Roche/Daiichi Sankyo). The arrival of these drugs, each of which demonstrated a survival benefit in clinical trials,2–4 has provided oncologists with new choices in a field in which options were slim and the prognosis grim. In a meta-analysis of 42 phase 2 trials enrolling 2,100 patients over the course of three decades, the median overall survival was 6.2 months, and after 1 year, only 25% of patients were still living.5
The National Comprehensive Cancer Network (NCCN)6 now lists ipilimumab and vemurafenib among the small number of preferred systemic regimens for treating advanced and meta-static melanoma; the others are high-dose interleukin-2 (IL-2) and medications that are given in a clinical trial.6 Other active regimens mentioned in the NCCN Guidelines are dacarbazine (DTIC-Dome, Bayer); temozolomide (Temodar, Schering/Merck); combination chemotherapy or biochemotherapy based on dacarbazine or temozolomide; imatinib (Gleevec, Novartis) for c-kit–mutated tumors; paclitaxel (Taxol, Bristol-Myers Squibb); and a combination of paclitaxel and carboplatin (Paraplatin, Bristol-Myers Squibb).
Ipilimumab is a fully human monoclonal antibody that promotes antitumor activity by blocking cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), which down-regulates pathways leading to T-cell activation. Ipilimumab and other immunomodulators in development were discussed in the September 2012 issue of P&T.7
In one phase 3 trial enrolling patients with previously untreated metastatic melanoma (N = 676), median overall survival was 10.0 months with ipilimumab plus glycoprotein 100 (gp100) versus 6.4 months in patients receiving gp100 alone.3 In another phase 3 trial of previously untreated metastatic melanoma (N = 502), ipilimumab plus dacarbazine provided a modest survival benefit compared with dacarbazine plus placebo for a median overall survival of 11.2 and 9.1 months, respectively.4
In both trials, the most common adverse events (AEs) were immune-related and were seen in 60% of patients receiving ipilimumab, compared with 32% of patients receiving gp100, and in 78% of the ipilimumab/dacarbazine group, compared with 38% of the dacarbazine/placebo group. These AEs can be so severe that they are mentioned in a boxed warning in the prescribing information for ipilimumab.1
Vemurafenib is an oral drug that inhibits the most common mutation of BRAF (V600E), found in about half of patients with metastatic melanoma. BRAF is a component in a MAPK (ERK) signaling pathway that culminates with activation of transcription factors important for cell growth, proliferation, and survival. Last year, encouraging phase 3 results were reported for two investigational small molecules, dabrafenib and trametinib (both from GlaxoSmithKline) that also interact with the same pathway.
Resembling vemurafenib, dabrafenib also inhibits mutated BRAF (V600E and V600K), whereas trametinib inhibits MEK, another component of the MAPK pathway downstream from BRAF. If dabrafenib, trametinib, and new immunomodulators gain FDA approval, the clinical challenge facing oncologists will not be to select the best immunomodulator or the best BRAF inhibitor but to devise the best therapeutic approach, which would probably involve sequential or simultaneous combinations of ipilimumab, vemurafenib, and emerging drugs. Indeed, after some of the world’s leading melanoma researchers met in Naples, Italy, in December 2011 to discuss new approaches to prevention, diagnosis, and treatment, they remarked, “Surely, the motto in melanoma therapy for [the] next years will be: combine, combine, combine!”8
This article describes the MAPK (ERK) pathway in melanoma and the various choices that oncologists and P&T committee members may soon face in light of emerging drugs that directly inhibit this pathway.
The MAPK Signaling Pathway
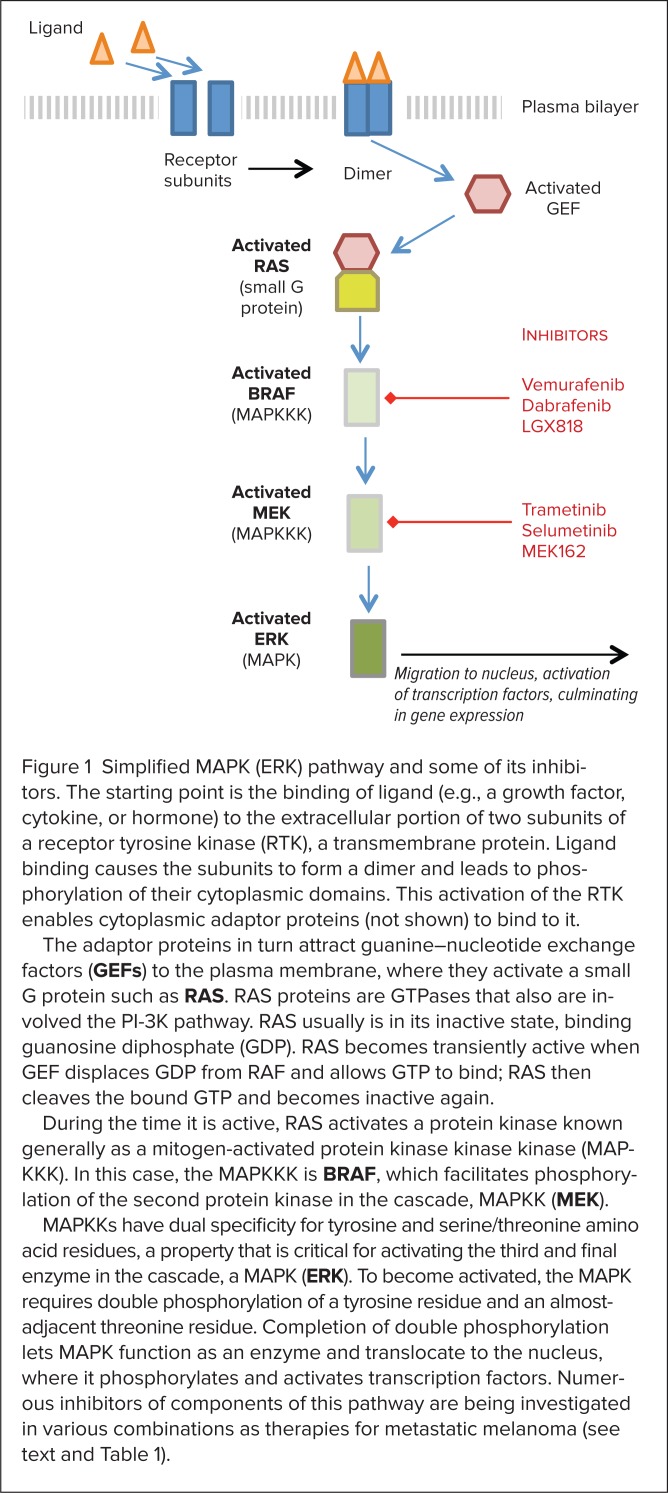
The RAS-RAF-MEK-ERK pathway is far more complex than once thought. Figure 1 presents a simplified description of its basic components. The pathway’s general structure includes a small G protein (RAS) and three protein kinases (RAF, MEK, ERK). (A kinase is an enzyme that catalyzes transfer of a phosphate group from a donor molecule to an acceptor.) The starting point for this pathway is the binding of ligand to a transmembrane protein, a receptor tyrosine kinase (RTK). The resulting signaling cascade culminates with translocation of ERK (MAPK) to the nucleus, where ERK activates transcription factors that result in gene expression.
Figure 1.
Simplified MAPK (ERK) pathway and some of its inhibitors. The starting point is the binding of ligand (e.g., a growth factor, cytokine, or hormone) to the extracellular portion of two subunits of a receptor tyrosine kinase (RTK), a transmembrane protein. Ligand binding causes the subunits to form a dimer and leads to phosphorylation of their cytoplasmic domains. This activation of the RTK enables cytoplasmic adaptor proteins (not shown) to bind to it.
The adaptor proteins in turn attract guanine–nucleotide exchange factors (GEFs) to the plasma membrane, where they activate a small G protein such as RAS. RAS proteins are GTPases that also are involved the PI-3K pathway. RAS usually is in its inactive state, binding guanosine diphosphate (GDP). RAS becomes transiently active when GEF displaces GDP from RAF and allows GTP to bind; RAS then cleaves the bound GTP and becomes inactive again.
During the time it is active, RAS activates a protein kinase known generally as a mitogen-activated protein kinase kinase kinase (MAPKKK). In this case, the MAPKKK is BRAF, which facilitates phosphorylation of the second protein kinase in the cascade, MAPKK (MEK).
MAPKKs have dual specificity for tyrosine and serine/threonine amino acid residues, a property that is critical for activating the third and final enzyme in the cascade, a MAPK (ERK). To become activated, the MAPK requires double phosphorylation of a tyrosine residue and an almost-adjacent threonine residue. Completion of double phosphorylation lets MAPK function as an enzyme and translocate to the nucleus, where it phosphorylates and activates transcription factors. Numerous inhibitors of components of this pathway are being investigated in various combinations as therapies for metastatic melanoma (see text and Table 1).
RAS proteins are GTPases (small G proteins) involved in the MAPK signaling cascade as well as the phosphatidylinositol-3-kinase (PI-3K) pathway. Mutations of RAS result in the loss of its enzymatic properties, allowing it to remain bound to guanosine triphosphate (GTP) and hence active.9 Among the three RAS proteins (HRAS, KRAS, and NRAS), all of which are frequently mutated in human cancers, only NRAS mutations are commonly encountered in melanoma (15%–20%). HRAS mutations in are found in fewer than 1% of melanomas, and KRAS mutations are rare.9
A glossary of terms used in genetics is presented in Table 1.
Table 1.
Terminology
| AZD6244. Selumetinib, a MEK1/2 inhibitor. |
| BRAF. One of the 3 Raf proteins found in mammals. First identified in 2002, BRAF mutations are found in different types of tumors, including 50% to 70% of human melanomas. The most common of these mutations is the substitution of glutamic acid (E) for valine (V) at position 600; this mutation is designated as V600E (formerly, V599E). |
| BREAK-3. Phase 3 study of dabrafenib in patients with the BRAF V600E mutation. Median progression-free survival (PFS) was 5.1 months in dabrafenib arm versus 2.7 months in dacarbazine arm. The risk of disease progression or death was reduced by 30% (hazard ratio, 0.30; P < 0.00001). |
| Codon. A sequence of three nucleotides in the genetic code that specifies a particular amino acid or a start or stop code. |
| CRAF. c-Raf is an enzyme that in humans is encoded by the RAF1 gene (also known as Raf-1); the first of three human RAF isoforms to be identified. |
| Dabrafenib (GSK 2118436). An oral inhibitor of BRAF V600E. Discovered and developed by GlaxoSmithKline. Positive results from a phase 3 trial, BREAK-3, were presented at the 2012 American Society of Clinical Oncology annual meeting. |
| ERK. Extracellular signal-regulated kinase. |
| GEF. Guanine–nucleotide exchange factor. This cytoplasmic protein is recruited to the inner cell membrane by adaptor proteins; it displaces guanosine diphosphate (GDP) from a small G protein like RAS, allowing GTP to bind to the small G protein and activate it. RAS then cleaves the bound GTP to GDP and reverts to its inactive state. |
| GDP. Guanosine diphosphate. |
| GTP. Guanosine triphosphate; is converted to GDP in signal transduction via action of a GTPase such as RAS. |
| HGF. Hepatocyte growth factor; is secreted by stromal cells in tumors and activates MET, a receptor tyrosine kinase (RTK). Activates both MAPK and PI-3K pathways and is implicated in resistance to BRAF inhibitors. Along with its receptor, HGF may offer a new therapeutic target in metastatic melanoma; to be used in combination with RAF inhibition. |
| Ipilimumab (Yervoy, Bristol-Myers Squibb); an infused immunomodulator approved by the FDA in 2011 for patients with advanced melanoma. This fully human monoclonal antibody blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), which down-regulates pathways leading to T-cell activation. |
| Isoform. Functionally similar proteins that have similar but not identical amino acid sequences and that are encoded by different genes or by RNA transcripts of the same gene from which different exons have been removed. |
| Kinase. An enzyme that catalyzes the transfer of a phosphate group from a donor, such as adenosine triphosphate (ATP) or adenosine diphosphate (ADP) to an acceptor (e.g., tyrosine). |
| LGX818 (Novartis). An orally available small molecule selective for BRAF V600E; is being investigated in combination with an inhibitor of MEK1 and MEK2 called MEK162. |
| KRAS. v-Ki-RAS-2 Kirsten rat sarcoma viral oncogene. |
| MAPK. Mitogen-activated protein kinase. Activated by a MAPKK, after which it translocates to the nucleus. Requires phosphorylation of both a tyrosine and a threonine amino acid residue in order to become activated and translocated, which only a MAPKK (e.g., MEK) can provide. See ERK. |
| MAP kinase cascade. Activates transcription factors. Cascade is initiated by a small G protein (e.g., NRAS), upon its activation by a guanosine–nucleotide exchange factor (GEF). The first enzyme activated is MAPKKKK, followed by MAPKK, and finally MAPK. |
| MAPKK. MAP kinase kinase; activated by MAPKKK and then activates MAPK. Has dual specificity for tyrosine and serine/threonine residues. See MEK. |
| MAPKKK = MAP kinase kinase kinase; an enzyme that activates MAPKK. See RAF. |
| MEK. Mitogen-activated ERK kinase, a family of seven human MAP kinase kinases, with dual specificity for tyrosine and serine/threonine residues. Components of four signaling pathways: ERK1/2 (MEK1/2), p38 (MEK3/6, and sometimes MEK4), JNK (MEK4/7), and ERK5 (MEK5). ERK1/2 are the only known substrates of MEK1/2. Kinase catalytic domains of MEK1 and MEK2 are 86% identical. |
| MEK162 (Novartis). The orally available inhibitor of MEK1 and MEK2; also known as ARRY-162. |
| MET. A transmembrane receptor for hepatocyte growth factor (HGF), its only known ligand; also called HGF receptor (HGFR). |
| METRIC. A phase 3 trial (NCT01245062) of trametinib; results were presented at the 2012 ASCO Annual Meeting. Enrolled were 322 patients with metastatic melanoma and a BRAF V600E or V600K mutation. Median progression-free survival (PFS) was 4.8 months with trametinib and 1.5 months with chemotherapy. |
| MK2206. An Akt inhibitor. |
| Mitogen. A substance that induces mitosis and cell transformation, especially in lymphocytes. |
| ORR. Objective/overall response rate; an endpoint in cancer trials often defined as the percentage of subjects with a confirmed complete response (CR) or partial response (PR) at any time per RECIST 1.1. |
| OS. Overall survival; an endpoint in cancer trials defined as time from randomization until death from any cause. |
| PFS. Progression-free survival; an endpoint in cancer trials and a surrogate endpoint for overall survival (OS). Often defined as the time from randomization until the earliest date of disease progression or death from any cause. |
| PI-3K. Phosphatidylinositol-3-kinase; a family of intracellular signaling molecules, regulated by phosphatase and tensin homologue (PTEN) in non-diseased cells. |
| PX-866 (Oncothyreon). A small molecule that irreversibly inhibits PI-3K. |
| RAF. A cytosolic protein kinase that directly activates MEK, following the activation of RAF by RAS. In addition to BRAF mutations, which are common in melanoma, ARAF (a-Raf) and CRAF (c-Raf) are the other two RAF proteins found in mammals. |
| RAS. A family of GTP-ases (NRAS, HRAS, KRAS). Small G proteins are embedded in the inner surface of cell membrane and are normally inactive and bound by GDP. Activated by exchange of GTP for GDP, either via external signal or oncogenic mutation, resulting in constitutive GTP-binding. Activated RAS interacts with members of the RAF family. |
| RECIST 1.1. Response Evaluation Criteria in Solid Tumors, version 1.1. |
| RTK. Receptor tyrosine kinase; a large group of cell–surface receptors. |
| Selumetinib (AZD6244 and ARRY-886). An oral small molecule that inhibits MEK1 and MEK2. Developed by Array BioPharma, licensed to AstraZeneca. |
| Small G protein. Monomeric G proteins (e.g., NRAS) that serve as intra-cellular signaling molecules. When active, they bind GTP and hydrolyze it to GDP, becoming inactive. It is distinct from the large G proteins consisting of three subunits. |
| Trametinib (GSK 1120212). An oral selective inhibitor of MEK1 and MEK2. Discovered by Japan Tobacco and licensed by GlaxoSmithKline in 2006. It was the first MEK inhibitor to demonstrate efficacy in a late-phase melanoma trial; the phase 3 trial was METRIC. |
| Vemurafenib (Zelboraf, PLX 4032, RG 7204, and RO 5185426, Roche/Daiichi Sankyo). This drug selectively binds to the ATP-binding site of the BRAF V600E mutation, present in about half of all melanomas (and in 8% of all solid tumors). |
| Yervoy. Ipilimumab. |
| Zelboraf. Vemurafenib. |
BRAF and BRAF Inhibitors
BRAF is one of three RAF proteins found in mammals. First identified in 2002, human BRAF mutations are found in many cancers.10 More than 50 point (single-base substitution) mutations have been found in BRAF, mostly in two regions of the kinase domain. Under normal conditions, these regions interact and keep the enzyme in its inactive state, absent a signal from RAS.11
The most common BRAF point mutation is one in which thymidine is replaced by adenosine at nucleotide 1796,10 leading to the substitution of glutamic acid (E) for valine (V) at the 600th amino acid residue in the expressed protein, hence the identification of this mutation as V600E. As a result of an error, V600E was initially designated as V599E and is identified as such in earlier literature.12
At the molecular level, the consequence of this mutation is that the two regions in the kinase domain are forced apart into their active conformation. This occurs because the glutamate residue is larger than the valine and it is also electrically charged (negatively) instead of being hydrophobic.11 In the BRAF V600K mutation, the second most common mutation in melanoma, valine is similarly replaced by a larger positively charged residue, lysine (K).
In vitro, BRAF V600E displays kinase activity about 500-fold greater than that of wild-type (normal) BRAF.11 BRAF V600E acquires the ability to constitutively stimulate downstream signaling independent of upstream signals from RAS. At the cellular level, BRAF V600E promotes the proliferation and transformation of melanocytes into melanoma cells.12
Shortly after the discovery of BRAF mutations and their role in melanoma, cancer researchers predicted that drug development probably would begin with inhibitors of V600E because it is the most common mutation—and that multiple BRAF inhibitors with different mechanisms of action would be desirable.12 That is the path that has been pursued.
The first BRAF inhibitor investigated in clinical trials in melanoma was sorafenib (Nexavar, Bayer), a nonselective inhibitor of many tyrosine kinases (such as BRAF) and of RTKs such as vascular epidermal growth factor receptor (VEGFR). Sorafenib was initially known as a potent inhibitor of CRAF and later was found to also inhibit wild-type BRAF and mutant (V600E) BRAF in vitro.13
Promising results were seen in the subset of melanoma patients in a phase 1/2 trial that included numerous tumor types; however, in a phase 3 trial that was restricted to 270 patients, the combination of sorafenib plus carboplatin and paclitaxel failed to improve progression-free survival and all other efficacy endpoints compared with chemotherapy alone.14
In a review by Davies et al., this failure raised suspicions that mutated BRAF was not a good therapeutic target.15 Testing positive for BRAF V600E or any other mutation of BRAF, however, was not among the eligibility criteria in this phase 3 trial of sorafenib.16 Subsequent phase 3 trials of vemurafenib and dabrafenib demonstrated that targeting mutated BRAF was indeed efficacious in treating metastatic melanoma.
Inhibition of CRAF eventually may emerge as another therapeutic approach, because in melanoma cell lines with mutated RAS but wild-type BRAF, the signaling switches from BRAF to CRAF.17 In addition, when BRAF inhibition is used, MAPK signaling can be re-established via CRAF if hepatocyte growth factor (HGF) is available, from stromal cells in the tumor, to activate its receptor, MET.18 However, such bypassing of BRAF would be thwarted if MEK inhibition is used concurrently.
Vemurafenib
Compared with dacarbazine chemotherapy, vemurafenib was found in a phase 3 trial to reduce the relative risk of overall and progression-free survival by 63% and 74%, respectively, in 675 treatment-naive patients with metastatic melanoma and the BRAF V600E mutation.2 Overall and progression-free survival were the co-primary endpoints.
At 6 months, overall survival rates were 84% and 64% in the vemurafenib and dacarbazine groups, respectively, with a hazard ratio (HR) of 0.37 and a 95% confidence interval (CI) of 0.26 to 0.55.2 Median progression-free survival rates were 5.3 and 1.6 months with vemurafenib and dacarbazine, respectively (HR, 0.26; 95% CI, 0.20–0.33).2 Cutaneous squamous cell carcinoma or keratoacanthoma developed in 18% of patients receiving vemurafenib. The lesions were excised, and no dose modifications were required. However, 38% of patients who received vemurafenib needed dose adjustments because of other AEs. In addition to cutaneous events, other common AEs in the vemurafenib group were arthralgia and fatigue.2
Dabrafenib
In BREAK-3, a phase 3 trial enrolling 250 previously untreated patients with unresectable stage III or IV melanoma with the BRAF V600E mutation, dabrafenib improved progression-free survival, the primary endpoint, by 70% compared with dacarbazine (HR, 0.30; 95% CI, 0.18–0.53; P < 0.0001).16 Median progression-free survival rates were 5.1 and 2.7 months, respectively, with dabrafenib (n = 187) and dacarbazine (n = 63). Serious AEs reported in the dabrafenib group were pyrexia (4%), squamous cell carcinomas (6%), and new primary melanomas (2%).
MEK and MEK Inhibitors
MEK is a MAPKK that activates a MAPK (ERK), the final kinase in the RAS-RAF-MEK-ERK signaling pathway in melanoma. Seven human MEKs are components in four signaling pathways: ERK1/2 (MEK1/2), p38 (MEK3/6, and sometimes MEK4), JNK (MEK4/7), and ERK5 (MEK5). In their kinase catalytic domains, MEK1 and MEK2 are 86% identical, which is why the MEK1/2 inhibitors developed thus far are not selective for either isoform.19 So far, the only known substrates for MEK1 and MEK2 are ERK1 and ERK2.
Trametinib
Trametinib (GSK 1120212) is a small-molecule oral inhibitor of MEK1 and MEK2. Results of METRIC, a phase 3 trial of trametinib in patients with advanced or metastatic melanoma and BRAF V600E/K mutations, were presented at the 2012 American Society of Oncology (ASCO) annual meeting20 and were simultaneously published online in June 2012.21
Patients were randomly assigned, in a 2:1 ratio, to receive trametinib once daily (n = 214) or chemotherapy with dacarbazine or paclitaxel (n = 108). In the primary population, consisting of the 85% of patients who were BRAF V600E–positive and did not have brain metastases at baseline (n = 273), median progression-free survival (the primary endpoint) was 4.8 months for trametinib and 1.4 months for chemotherapy (HR, 0.44 [95% CI, 0.31–0.64; P < 0.0001]). In the intent-to-treat (ITT) population (N = 322), results were similar (HR, 0.45; 95% CI, 0.33–0.63).21
The only patients who did not benefit from trametinib therapy were those with the BRAF V600K mutation and those 65 years of age and older. Progression-free survival was chosen as the primary endpoint because of the concern that the subjects’ post-protocol use of ipilimumab or vemurafenib might confound an overall survival endpoint.21 Overall response rates (ORRs) were 24% and 7%, respectively, in the trametinib and chemotherapy groups.
MEK162
Made by Novartis, MEK162 is an oral small molecule that inhibits MEK1 and MEK2. It is being investigated in combination with a selective BRAF inhibitor (LGX818, Novartis), for patients with metastatic melanoma.
Combination Therapy
Some patterns have emerged with the use of BRAF inhibitors and CTLA-4 inhibitors in metastatic melanoma. With CTLA-4 inhibition, the onset of response is slow; in a minority of patients, however, the response is longer in duration. By contrast, with BRAF inhibition, the onset of response tends to be rapid and the rate of response is high, yet the duration of response is limited. Resistance to BRAF inhibitors often develops within a few months after treatment begins, not because of new BRAF mutations but often because of the emergence of mutations in other genes (notably NRAS and MEK) that restore RAS-RAF-MEK-ERK signaling.22
Another possible mechanism of resistance is redundancy of RTK-transduced signaling in cancer cells.23 One such RTK is MET, the receptor for HGF. It has been discovered that secretion of HGF by stromal cells in tumors activates MET and both the MAPK and PI-3K signaling pathways, thereby conferring resistance to RAF inhibitors.18 This finding suggests that dual inhibition of BRAF and HGF or its receptor may be fruitful, but this approach has not yet been explored in metastatic melanoma.
There is also keen interest in exploring various combinations to see whether the combination of BRAF inhibition and CTLA-4 inhibition creates synergy and whether simultaneous targeting of BRAF and MEK is beneficial. In a phase 1/2 trial, the combination of dabrafenib and trametinib has shown encouraging clinical activity in patients with BRAF V600-mutated metastatic melanoma,24 and numerous clinical trials are in progress to test this combination and other combinations of BRAF and MEK inhibitors (Table 2).
Table 2.
Selected Trials of Combination Therapies Involving the MAPK (ERK) Pathway in Patients With Advanced Melanoma
| National Clinical Trial Code Identifier (Sponsor) | Drug | Phase (No. of Subjects) | Stage | Primary Endpoint (Secondary) | Start Date Primary Completion Date Completion Date |
|---|---|---|---|---|---|
| NCT01597908 (GlaxoSmithKline) | Dabrafenib (BRAFi) + trametinib (MEKi) vs. vemurafenib (BRAFi) | 3 (694) | Stage IIIc or IV, BRAF V600E/K+ | OS (PFS, ORR, response duration) | June 2012 March 2014 June 2015 |
| NCT01584648 (GlaxoSmithKline) | Dabrafenib + trametinib vs. dabrafenib | 3 (340) | Stage IIIc or IV, BRAF V600E/K+ | PFS (OS, ORR, response duration) | May 2012 May 2013 May 2015 |
| NCT01495988 (Genentech) | Vemurafenib + bevacizumab (VEGFi) vs. vemurafenib | 2 (180) | Stage IV, BRAF V600+ | PFS (OS, RR, response duration) | June 2012 September 2014 March 2015 |
| NCT01510444 (National Cancer Institute, Moffitt Cancer Center) | Selumetinib (MEKi) + MK2206 (Akt inhibitor) vs. selumetinib | 2 (72) | Stage III or IV, BRAF V600+; failure of selective BRAFi | PFS (OS) | Jan 2012 April 2014 April 2014 |
| NCT01519427 (National Cancer Institute, Moffitt Cancer Center) | Selumetinib + MK2206 vs. selumetinib | 2 (72) | Stage III or IV, BRAF V600+; failure of vemurafenib or dabrafenib | PFS (OS, ORR) | Jan 2012 July 2012 |
| NCT01543698 (Novartis) | LGX818 (BRAFi) +MEK162 (MEKi) | 1b/2 (87) | Stage IIIb to IV, BRAF V600+ | Phase 1b: MTD Phase 2: DCR, ORR (PFS) |
May 2012 March 2015 March 2015 |
| NCT01400451 (Bristol-Myers Squibb) | Vemurafenib + ipilimumab (CTLA-4 inhibitor) | 1/2 (50) | Metastatic melanoma, BRAF V600+ | Phase 1: Safety and tolerability Phase 2: OS |
November 2011 August 2015 August 2015 |
| NCT01616199 (Oncothyreon) | PX-866 (PI-3K inhibitor) + vemurafenib | 1/2 (146) | Stage IIIc or IV, BRAF V600E/K+ | Phase 1: AEs Phase 2: PFS (ORR, DCR) |
May 2012 December 2104 March 2015 |
AE = adverse event; Akt = protein kinase B; BRAFi = BRAF inhibitor; CTLA-4 = cytotoxic T-lymphocyte antigen 4; DCR = disease control rate; MEKi = MEK inhibitor; MTD = maximum tolerated dose; OS = overall survival; ORR = objective/overall response rate; PFS = progression-free survival; VEGFi = vascular epidermal growth factor inhibitor.
The most advanced of these studies are two phase 3 trials. Following the positive phase 3 trial results of single-agent treatment with dabrafenib and trametinib versus chemotherapy, these two trials are now pitting the combination of dabrafenib and trametinib against single-agent treatment with vemurafenib (NCT01597908) or dabrafenib (NCT01584648).
Sequential Ipilimumab and BRAF Inhibitors
Combination therapy with targeted agents is already a possibility for patients with metastatic melanoma, as a result of the FDA’s recent approvals of ipilimumab and vemurafenib. Although vemurafenib is restricted to patients with the BRAF V600E mutation, patients who are BRAF V600E–positive also may be candidates for ipilimumab. Some experts suggest that for appropriate patients, vemurafenib and ipilimumab would work better used sequentially instead of as monotherapy.25 The question is, in which patients and in which sequence?
A retrospective study by Ascierto et al. identified 34 patients who had received vemurafenib or dabrafenib in phase 3 trials and also had received ipilimumab before or after the BRAF inhibitor.25 Twenty-eight patients received a BRAF inhibitor first (vemurafenib, 12; dabrafenib, 16), and six patients received ipilimumab before a BRAF inhibitor (vemurafenib, 4; dabrafenib, 2).
In both groups, the second agent was administered upon the emergence of disease progression with the first. All six subjects who received ipilimumab before the BRAF inhibitor had received ipilimumab as second-line therapy, whereas half the patients who received a BRAF inhibitor before ipilimumab had had previous treatment, predominantly chemotherapy. At the time of the analysis, all six subjects who received ipilimumab, followed by a BRAF inhibitor, were still living (median follow-up, 11.2 months).
Among the 28 patients who began with a BRAF inhibitor, 12 experienced rapid disease progression and died before all four induction doses of ipilimumab could be given (median overall survival, 5.7 months). However, 16 patients with slower disease progression completed ipilimumab induction therapy (median overall survival, 18.6 months; P < 0.00001).
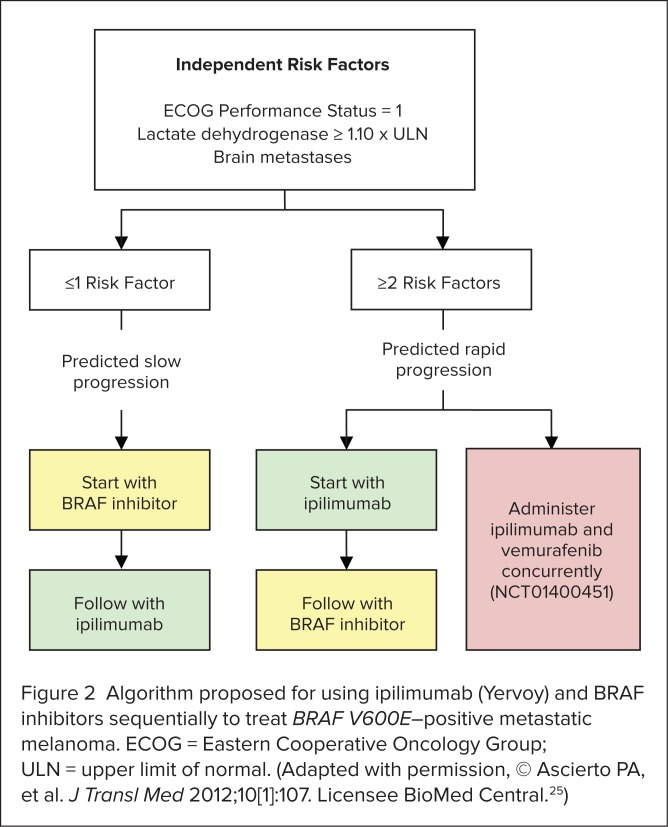
After analyzing patients’ baseline factors, the researchers identified three independent risk factors (Figure 2) that could be used to predict whether a patient was likely to have slowly progressing disease (and thus be able to complete ipilimumab induction therapy) or would be likely to experience rapid disease progression (and thus should start treatment with ipilimumab). Applying these risk factors, they suggested an algorithm for determining the sequence of ipilimumab and BRAF inhibitors (see Figure 2), noting that further studies enrolling larger numbers of patients are needed to validate the concept.
Figure 2.
Algorithm proposed for using ipilimumab (Yervoy) and BRAF inhibitors sequentially to treat BRAF V600E–positive metastatic melanoma. ECOG = Eastern Cooperative Oncology Group; ULN = upper limit of normal. (Adapted with permission, © Ascierto PA, et al. J Transl Med 2012;10[1]:107. Licensee BioMed Central.25)
The authors noted that in their retrospective study, patients had been switched to ipilimumab or a BRAF inhibitor after disease progression was documented, and they wondered whether better outcomes would occur if the switch were made at the point when disease control was achieved. They also speculated that patients at risk of rapid disease progression might benefit more from concurrent treatment with ipilimumab and dabrafenib rather than from sequential treatment. This hypothesis is being tested in a clinical trial, now in progress (NCT01400451).
Other Possible Combinations
In a few years, the question of whether a BRAF inhibitor and ipilimumab should be given sequentially or concurrently, if at all, is likely to become more complicated. Phase 3 trials now under way should be able to demonstrate whether concurrent inhibition of BRAF and MEK with dabrafenib and trametinib works better than single-agent therapy with dabrafenib or vemurafenib.
Yet these trials will not answer the question of whether dabrafenib is superior to vemurafenib, or to LGX818, for that matter. An oral drug selective for BRAF V600E (LGX818) is being investigated in Clinical Trial NCT01543698 in combination with an inhibitor of MEK1 and MEK2 (MEK162). Novartis claims that the potency of LGX818 is 50-fold greater than that of other BRAF inhibitors.
Nor will any of the current trials show whether MEK162, trametinib, or selumetinib is the best choice among MEK inhibitors. If the investigational BRAF inhibitors and MEK inhibitors eventually reach the market (see Table 2), oncologists may be faced with nine different combinations even apart from combinations that could involve AKT or PI-3K inhibitors.
Activation of the PI-3K–AKT pathway, as a consequence of RTK overexpression, has been proposed as another mechanism of acquired resistance to BRAF inhibition.26 Signals along this pathway promote cell survival in a manner redundant to signaling along the MAPK pathway.26
Looking even further ahead, a drug that blocks the P-glycoprotein ABCB5 transporter could be developed for use in conjunction with vemurafenib and other BRAF inhibitors.27 ABCB5 is a protein believed to be involved in the ejection of cytotoxic drugs from the cytoplasm; BRAF V600E cell lines that are ABCB5-positive survive doses of vemurafenib that kill V600E cells that are ABCB5-negative. Likewise, as mentioned previously, BRAF inhibition could be combined with inhibition of HGF or its receptor, MET, to thwart another mechanism of resistance to BRAF inhibition.
Conclusion
In a substantial proportion of patients with metastatic melanoma, a point mutation (V600E) of the BRAF gene is present in the tumor. As a consequence, the RAS-RAF-MEK-ERK signaling pathway is transformed from one that is transiently activated, in response to ligand binding, to one that functions constantly because of constitutive activation, which promotes tumor proliferation and survival. In such patients, inhibiting BRAF with vemurafenib has been shown to provide a survival benefit.
Even though a high percentage of patients respond to vemurafenib, the duration of response tends to be short because of emerging mutations elsewhere along the signaling pathway and other mechanisms of resistance. Combination therapy that provides simultaneous inhibition of BRAF and MEK or treatment that adds sequential or concurrent CTLA-4 inhibition (i.e., ipilimumab) may prove to be a better approach than the use of BRAF inhibition alone.
In summary, it is unlikely that any of the numerous clinical trials in progress will be able to determine which combination under investigation is the best therapy for patients with BRAF-mutated metastatic melanoma. However, any combination that demonstrates a survival benefit that is comparable or superior to that of vemurafenib or ipilimumab is likely to be added to the NCCN’s short list of preferred systemic treatments for this disease. Mindful of the expense and limitations of ipilimumab and vemurafenib, P&T committee members will want to be on the lookout for combination therapies that offer the highest value.
References
- 1.Yervoy (ipilimumab), package insert) Princeton N.J.: Bristol-Myers Squibb; Mar, 2011. [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 5.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) Melanoma. Version 1.2013. NCCN Clinical Practice Guidelines in Oncology. Available at www.nccn.org. Accessed October 9, 2012.
- 7.Fellner C. Ipilimumab (Yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. P&T. 2012;37(9):503–511. 530. [PMC free article] [PubMed] [Google Scholar]
- 8.Ascierto PA, Grimaldi AM, Curti B, et al. Future perspectives in melanoma research. Meeting report from Melanoma Research: A Bridge from Naples to the World. Napoli, December 5–6, 2011. J Transl Med. 2012;10(1):83. doi: 10.1186/1479-5876-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daud A, Bastian BC. Beyond BRAF in melanoma. Curr Top Microbiol Immunol. 2011 Aug 9; doi: 10.1007/82_2011_163. (online) [DOI] [PubMed] [Google Scholar]
- 10.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 11.Wan PT, Garnett MJ, Roe SM, et al. Cancer Genome Project. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 12.Garnett MJ, Marais R. Guilty as charged: B-RAF is a human oncogene. Cancer Cell. 2004;6(4):313–319. doi: 10.1016/j.ccr.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad-spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 14.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27(17):2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]
- 15.Davies MA, Gershenwald JE. Targeted therapy for melanoma: A primer. Surg Oncol Clin North Am. 2011;20(1):165–180. doi: 10.1016/j.soc.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 17.Dumaz N, Hayward R, Martin J, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66(19):9483–9491. doi: 10.1158/0008-5472.CAN-05-4227. [DOI] [PubMed] [Google Scholar]
- 18.Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487(7408):500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frémin C, Meloche S. From basic research to clinical development of MEK1/2 inhibitors for cancer therapy. J Hematol Oncol. 2010;3:8. doi: 10.1186/1756-8722-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robert C, Flaherty KT, Hersey P, et al. METRIC phase III study: Efficacy of trametinib (T), a potent and selective MEK inhibitor (MEKi), in progression-free survival (PFS) and overall survival (OS), compared with chemotherapy (C) in patients (pts) with BRAF V600E/K mutant advanced or metastatic melanoma (MM) (Abstract LBA8509) J Clin Oncol. 2012;30(Suppl) [Google Scholar]
- 21.Flaherty KT, Robert C, Hersey P, et al. METRIC Study Group Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012 Jun 4; doi: 10.1056/NEJMoa1203421. (online) [DOI] [PubMed] [Google Scholar]
- 22.Greger JG, Eastman SD, Zhang V, et al. Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther. 2012;11(4):909–920. doi: 10.1158/1535-7163.MCT-11-0989. [DOI] [PubMed] [Google Scholar]
- 23.Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487(7408):505–509. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber JS, Flaherty KT, Infante JR, et al. Updated safety and efficacy results from a phase I/II study of the oral BRAF inhibitor dabrafenib (GSK2118436) combined with the oral MEK 1/2 inhibitor trametinib (GSK1120212) in patients with BRAFi-naive metastatic melanoma (Abstract 8510) J Clin Oncol. 2012;30(Suppl) [Google Scholar]
- 25.Ascierto PA, Simeone E, Giannarelli D, et al. Sequencing of BRAF inhibitors and ipilimumab in patients with metastatic melanoma: A possible algorithm for clinical use. J Transl Med. 2012;10(1):107. doi: 10.1186/1479-5876-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo RS. Combinatorial therapies to overcome B-RAF inhibitor resistance in melanomas. Pharmacogenomics. 2012;13(2):125–128. doi: 10.2217/pgs.11.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chartrain M, Riond J, Stennevin A, et al. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One. 2012 2012 May 24;7(5) doi: 10.1371/journal.pone.0036762. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]