Abstract
Identification of the serrated neoplasia pathway has improved our understanding of the pathogenesis of colorectal cancer (CRC). Insights have included an increased recognition of the malignant potential of different types of serrated polyps, such as sessile and traditional serrated adenomas. Sessile serrated adenomas share molecular features with colon tumors, such as microsatellite instability and a methylator phenotype, indicating that these lesions are precursors that progress via the serrated neoplasia pathway. There is evidence that the serrated pathway contributes to interval or missed cancers. These data have important implications for clinical practice and CRC prevention, since hyperplastic polyps were previously regarded as having no malignant potential. Endoscopic detection of serrated polyps is a challenge because they are often inconspicuous with indistinct margins, and are frequently covered by adherent mucus. It is important for gastroenterologists to recognize the subtle endoscopic features of serrated polyps, which would facilitate their detection and removal, to ensure a high-quality colonoscopy examination. Recognition of the role of serrated polyps in colon carcinogenesis has led to the inclusion of these lesions in post-polypectomy surveillance guidelines. However, an enhanced effort is needed to identify and completely remove serrated adenomas, with the goal of increasing the effectiveness of colonoscopy to reduce CRC incidence.
Keywords: Serrated Polyps, Sessile Serrated Adenomas, Serrated Polyposis Syndrome, Colon Cancer
I. Introduction
Colorectal carcinoma (CRC) is the most common gastrointestinal malignancy worldwide1, and most cases originate from identifiable precursor lesions. Traditionally, epithelial polyps of the colorectum were classified as either hyperplastic or adenomatous with the adenomatous polyps representing the principal precursor to CRCs. However, advances in the molecular understanding of CRC suggest it is a heterogeneous disorder arising via multiple pathways including the serrated pathway, whereby the serrated polyp is the precursor lesion. In this pathway, a distinct subtype of serrated polyps [known as sessile serrated adenomas (SSA/Ps)] has become recognized as an important contributor to CRC incidence2. Serrated polyps have distinct histopathological features. Their often subtle appearance at endoscopy poses challenges for endoscopic detection and removal, which are critical for CRC prevention. In this manuscript, we review the classification of serrated polyps, the molecular characteristics of the serrated neoplasia pathway, and the detection and appropriate management of these lesions.
II. Pathology of Serrated Polyps
Classification
Serrated polyps are heterogeneous lesions characterized histologically by glandular serration, i.e., a “saw-tooth” infolding of colonic crypt epithelium. This feature is believed to be the result of increased cell turnover combined with delayed migration or failure of apoptosis at the mucosal surface leading to an accumulation of epithelial cells that are accommodated by infolding (serration) of the epithelial crypt lining3. Historically, polyps with serrated architecture were considered indolent, hyperproliferative, non-neoplastic hyperplastic polyps. It is now recognized that several distinct subtypes of serrated polyps exist, and a subset may progress to invasive cancer through a serrated neoplasia pathway. Serrated polyp nomenclature is in evolution. The most recent classification by the World Health Organization (WHO) categorizes them into two main groups based on the presence or absence of dysplasia (Table 1). This includes serrated polyp subtypes: hyperplastic polyps, sessile serrated adenoma/polyp (SSA/P), SSA/P with cytological dysplasia, and traditional serrated adenoma (TSA).4
Table 1.
WHO Classification of Serrated Polypsa
| Non-dysplastic | Dysplastic |
|---|---|
Hyperplastic polyps:
|
Sessile serrated adenoma/polyp (SSA/P) with dysplasia |
| Sessile serrated adenoma/polyp (SSA/P) | Traditional serrated adenoma (TSA) |
See Snover et al.4
Hyperplastic Polyp (HP)
The defining histologic feature of HPs is a sawtooth pattern of epithelial infolding in the upper half of the crypt with a lack of cytologic dysplasia (Figure 1A). HPs are subclassified into microvesicular, goblet cell-rich, and mucin-poor variants3. The microvesicular type is most frequent type and is characterized by epithelial cells with small droplets of cytoplasmic mucin and decreased goblet cells. There is abundant serration in the upper portion of the crypt, but the crypt base is straight. The microvesicular subtype often has mutation in the BRAF oncogene, suggesting these could be precursors to SSA/P5. In contrast, goblet cell-rich HPs have abundant goblet cells, less superficial serration and lack BRAF mutations. The rare mucin-poor HP, nearly devoid of cytoplasmic mucin, may have increased nuclear atypia. It remains unclear as to whether histologic subtyping of HPs has clinical utility and therefore, current guidelines advise against doing so in routine clinical practice.
Figure 1.
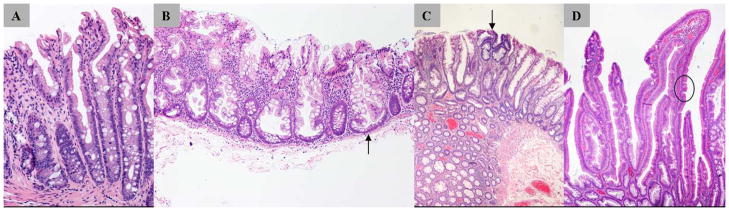
Histomicrographs of serrated polyps. A, Hyperplastic polyp (microvesicular subtype) with serrations in the upper half of the crypt and no cytologic dysplasia. B, Sessile serrated adenoma/polyp (SSA/P) with characteristic branching crypt bases (arrow). C, SSA/P with dysplasia: arrow indicates dysplastic epithelium with hyperchromatic nuclei and pseudostratification. D, Traditional serrated adenoma showing ectopic crypt (arrow and circle).
Sessile serrated adenoma/polyp (SSA/P)
SSA/Ps, like hyperplastic polyps, have serrated crypts but the SSA/P crypts are distorted with widened, branching bases that are a typical feature (Figure 1B)3. Dysplasia, seen in conventional adenomas, is not a feature of SSA/Ps, but focal dysplasia may develop during tumor progression3, 6. The presence of typical dysplasia classifies the polyp as SSA/P with dysplasia (Figure 1C), which may represent an intermediary in molecular progression of SSA/P to malignancy7. Colectomy specimens from CRC patients show serrated polyps more frequently with tumors showing microsatellite instability (MSI)8. These data were often obtained before recognition of SSA/Ps as a distinct entity, and re-review of the histology has suggested that many of these lesions were SSA/Ps9. When a remnant SSA/P is found adjacent to carcinoma, a transition zone of dysplasia is frequently present9. Molecular profiling of SSA/Ps indicates that dysplastic areas are likely the immediate precursors of CRCs that show MSI10.
Traditional serrated adenoma (TSA)
TSAs are also serrated, but have villiform projections lined by hypereosinophilic cells11. Premature crypt formation perpendicular to the longitudinal axis of the villus, is a characteristic histologic feature known as ectopic crypt formation, whereby the crypts have lost anchoring to the underlying muscularis mucosae11, 12 (Figure 1D). In some TSAs, cytologic dysplasia is present, but in others, pale pink cells with minimal cytologic changes represent metaplastic, non-proliferating senescent cells exist3, 9. The TSA is a poorly understood histologic entity. The molecular characterization of TSAs has shown polyps with either KRAS or BRAF mutations that suggest distinct variants despite overlapping morphologic features13.
III. Molecular Features of the Serrated Neoplasia Pathway
Most CRCs develop from conventional adenomas through a molecular pathway characterized chromosomal instability (CIN). However, approximately 15% of CRCs develop through an alternate pathway characterized by defective DNA MMR that gives rise to high frequency MSI (MSI-H). MSI was first described in association with Lynch syndrome (hereditary nonpolyposis colorectal cancer - HNPCC) due to germ-line mutations in MMR genes. In sporadic CRCs, MSI is a consequence of defective MMR due to hypermethylation of the MLH1 MMR gene that frequently occurs in a background of increased methylation of CpG islands in gene promoter regions known as the CpG island-methylator phenotype (CIMP)14–16. CIMP is not exclusive to MSI tumors since it is infrequently found in tumors lacking MLH1 methylation or MSI17. Thus, CIMP CRCs include almost all sporadic cases with MSI and a proportion of CRCs that are microsatellite stable (MSS)15, 17, 18. About 40% of CRCs with MSI and MLH1 hypermethylation carry hotspot mutations (V600E) in codon 15 of the BRAF oncogene17, 19. Mutations in BRAF result in activation of the mitogen-activated protein kinase (MAPK) pathway that promotes cell proliferation and survival.
The improved histological classification of serrated polyps has enabled a comparison of molecular features based upon polyp subtype. It is believed that most CRCs with the CIMP phenotype evolve through the serrated neoplasia pathway20, 21. A clear link has been found between serrated polyps, especially SSA/Ps, and sporadic CRCs showing MSI and CIMP18. Using a specific marker panel18, CIMP was detected in 7% of microvesicular HPs and in 48% of advanced serrated polyps22. CIMP positivity is frequent in proximal SSA/Ps,9 and SSA/P histology has been seen at the margins of MSI CRC s9, 23. If hypermethylation inactivates MLH1 early in the serrated pathway, then an MSI-H CRC will result.
BRAF and KRAS mutations are early molecular alterations in serrated lesions24 and are mutually exclusive in colorectal neoplasms16. A simplified diagram of these two serrated pathways is shown in Figure 2. CIMP has been shown to be strongly associated with point mutations in BRAF25. BRAF mutation is an early event in the serrated pathway being detected frequently in microvesicular HPs and in most SSAs that also display a high level of CIMP5. In contrast, BRAF mutations are not found in conventional adenomas25. Importantly, neither BRAF mutations nor CIMP are found in CRCs with germline MMR mutations that result in Lynch Syndrome, thereby, highlighting their association with the serrated pathway. Taken together, these findings support the notion that SSA/Ps are the precursor lesions of sporadic MSI CRC. While this end-point does intersect with the end-point in Lynch syndrome, it is important to remember that the major precursor lesion of CRCs in Lynch syndrome is the adenoma, not a serrated polyp.
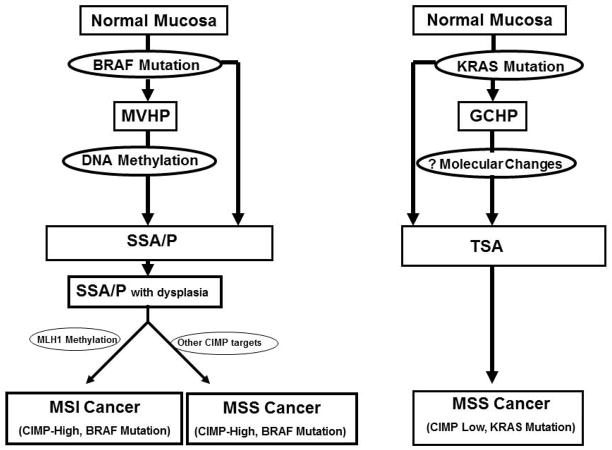
Figure 2.
Diagram of two potential molecular pathways of serrated neoplasia. The precursors of the first pathway are the microvesicular hyperplastic polyp and/or the sessile serrated adenoma/polyp (SSA/P) with the potential for SSA/P to arise de novo from normal mucosa. This pathway results in cancers that are CIMP-high and carry BRAFV600E mutations with either MSI or MSS status. The second pathway is less defined with the potential precursor lesion being the goblet cell hyperplastic polyp and the tradiational serrated adenoma (TSA). The end result of the second path is MSS and CIMP-low cancers that are associated with KRAS mutations, although this remains speculative. Abbrev.: CIMP, CpG Island methylator phenotype; MSI, microsatellite instability; MSS, microsatellite stable;
Compelling evidence supports a serrated neoplasia pathway with SSA/Ps bearing BRAF mutations as precursors of MSI-H and/or CIMP-high CRCs5, 25. A second pathway involves CIMP-low and MSS cancers that are associated with KRAS mutations. A precursor lesion of this second pathway may be the TSA. An additional feature of this second putative serrated pathway is silencing of the DNA repair gene methylguanine methyltransferase (MGMT) by promoter hypermethylation which has been associated with KRAS mutation and CIMP-low status26, 27. Further support for at least two serrated neoplasia pathways is the finding that KRAS and BRAF mutations segregate with lesion type, i.e., polypoid vs flat, respectively28. KRAS mutations are common in rectal and polypoid TSAs29, but are rare in SSA/Ps25. It is believed that some microsatellite stable (MSS) adenocarcinomas may originate from TSAs. In aggregate, data suggest that approximately 30% of colonic adenocarcinomas derive from the serrated neoplasia pathway10.
IV. Epidemiology of Serrated Polyps
By far, the most common serrated polyp is the conventional hyperplastic polyp which accounts for 70–95% of all serrated polyps30, 31 and most frequently occurs in the recto-sigmoid colon31. SSA/Ps account for 5–25% of serrated polyps and occur predominantly in the proximal colon30–33. While the prevalence of SSA/Ps varies depending on the clinical study, the overall the prevalence varies from 2% – 9%30–33. However, a recent study evaluating the prevalence of serrated polyps in the proximal colon in average-risk individuals undergoing screening colonoscopy found the prevalence of SSA/Ps to be as high as 20%34. TSAs are much less common than SSA/Ps and account for only < 1% of all colorectal polyps30, 31. TSAs typically occur in the distal colon and rectum, and tend to have a pedunculated or broad-based polypoid growth pattern when compared with SSA/P29.
Although uncommon, TSAs are likely precursor lesions to some CRCs as suggested by Longacre and Feniglio-Preiser who showed that 11% of TSAs contain intramucosal carcinoma35.
The primary risk factors for serrated neoplasia are common to subjects with conventional adenomas and include the combined effects of inherited (genetic) susceptibility and environmental factors. Environmental factors that increase the risk for serrated neoplasia include lifestyle and diet. In a study by Wallace et al, obesity, dietary fat, cigarette smoking, total energy intake, and red meat were associated with an increased risk of serrated polyps in the left colon; whereas, a family history of polyps and folate treatment were associated with an increased risk of serrated polyps in the proximal colon36. Cigarette smoking is associated with an increased risk of conventional adenomas and CRC. Studies examining the association of cigarette smoking and incident CRC have found that smokers have a significantly higher risk of tumors with MSI, CIMP, and BRAF mutations37. These molecular features, characteristic of SSA/Ps, suggest that cigarette smoking increases CRC risk via the serrated neoplasia pathway.
Jeevaratnam et al.38 first described the possibility of a familial serrated polyposis syndrome in 1996. Subsequently, cases of hyperplastic polyposis with synchronous adenocarcinoma were reported and it is now recognized that individuals with hyperplastic polyposis present with synchronous cancers of the colorectum in up to 25– 50% of cases26, 39–43. Recently, the term ‘hyperplastic polyposis’ has been changed to ‘serrated polyposis’ since a spectrum of serrated lesions, not just hyperplastic polyps41, can be found in this condition as discussed below.
V. Serrated Polyposis Syndrome
The recently modified WHO clinical criteria for the diagnosis of serrated polyposis includes any one of the following: 1) at least five histologically diagnosed serrated polyps proximal to the sigmoid colon, two of which are greater than 1 cm in diameter, or 2) any number of serrated polyps occurring proximal to the sigmoid colon in an individual having a first-degree relative with serrated polyposis, or 3) more than 20 serrated polyps of any size but distributed throughout the colon4. These criteria suggest that serrated polyposis may encompass a group of diseases rather than being a single entity, or represent a disease continuum.
The exact prevalence of SPS is unknown and it is under-recognized by endoscopists given lack of familiarity with SPS criteria and the need for more consistent application of such criteria in individual patients44. The mean age at diagnosis of SPS is 52 years39, 45, 46; however, the age at diagnosis is quite variable among studies41, 47. Approximately 5% of patients with SPS have at least one first-degree family member with the condition41, 46. Autosomal dominant and recessive inheritance of SPS has been suggested38, 41, 43, 48 with a 5-fold increased risk of CRC noted in first-degree relatives of SPS patients39. Further evidence that SPS has a heritable component is the finding that both first- and second-degree relatives of index patients are at increased risk of CRC, and that first-degree relatives are at significantly increased risk of pancreatic cancer49. Despite these observations, an underlying genetic basis for SPS has yet to be defined. Molecular analysis of serrated neoplasms from patients with SPS reveals the presence of CIMP as well as BRAF mutations that reflect the serrated pathway24, 31, 50. Interestingly, patients with MUTYH-associated polyposis can have a similar colonic phenotype as SPS with the finding of concomitant adenomas and serrated neoplasms51. However, serrated polyps are molecularly distinct from those found in patients with MUTYH-associated polyposis in that they show KRAS mutations, but lack CIMP and BRAF mutations51.
VI. Clinical Implications
The recognition of a serrated neoplasia pathway to CRC has important clinical implications for detection, surveillance, and treatment. While management of colonic serrated neoplasia should be based on the natural history and malignant potential of the various subtypes of serrated polyps, such data are limited as studies were performed prior to the distinction among serrated polyp subtypes. A critical yet unanswered question is whether certain serrated polyp subtypes, especially SSA/Ps, can progress to invasive cancer at a rate that is similar to or more rapid than conventional adenomas.
Studies by Jass et al52 suggested that progression via the serrated pathway can rapidly lead to malignant transformation. Potential for rapid malignant transformation was suggested by a review of 8 cases of invasive MSI CRCs and high-grade dysplasia arising from SSA/Ps (less than 1cm) showing cytologic dysplasia in the proximal colon53. In addition, this study found the interval from SSA/P to adenocarcinoma was less than 3 years in 18% and 3–6 years in 27% of cases. Lazarus et al54 showed a higher growth rate and rate of recurrence for SSA/Ps compared to conventional adenomas, suggesting that this serrated subtype may be more aggressive. In contrast, a recent and large cross-sectional study reported that the median age of patients with SSA/Ps was 61 years and those with serrated cancers was 76 years, suggesting a more indolent behavior of these serrated neoplasms32. It is known that the epigenetic inactivation of MLH1 in sporadic MSI CRCs is associated with older age at diagnosis55. Inactivation of MLH1 occurs in precursor SSA/Ps and these lesions are prone to acquire additional mutations56, 57. It is important to make the distinction between age at diagnosis and rapidity of progression to carcinoma.
Interval cancers, i.e., cancers occurring after a previous colonoscopy, represent either missed cancers, interval development of cancer due to missed polyps, incompletely removed polyps, or rapid progression of serrated polyps to cancer. Importantly, interval (or missed) cancers have been shown to be four times more likely as non-interval cancers to show MSI and CIMP which are both molecular signatures of the serrated neoplasia pathway21, 58. These data suggest that the serrated pathway may contribute disproportionately to interval or missed cancers. Evidence also suggest a greater risk of synchronous and metachronous neoplasia including CRC in individuals with SSA/Ps. In this regard, Schreiner et al showed that the presence of a proximally located or large (>10mm) SSA/Ps was an independent risk factor for the presence of CRC. In addition, the presence of large serrated polyps increases the risk for CRC, especially in the proximal colon7, 59. These data indicate the importance of removing all serrated lesions, with the exception of diminutive hyperplastic polyps in the rectosigmoid colon.
VII. Detection of Serrated Polyps
Serrated polyps are less likely than conventional adenomas to bleed, so fecal occult blood testing may not detect these lesions60. While there no data regarding the sensitivity and specificity of computed tomographic colonography to detect serrated polyps, the sessile and flat morphology of these lesions suggest that that this modality would perform poorly. Optical colonoscopy appears to the best of the current screening methods to detect serrated polyps60; however, significant challenges remain. Colonoscopy with polypectomy has been shown to significantly reduce the incidence of CRC; however, recent data indicate that it is substantially less effective at preventing proximal colon cancers61–64. Furthermore, interval CRCs are three times more likely than non-interval cancers to occur in the proximal colon and four times more likely to show MSI58. Failure to recognize and adequately remove neoplastic polyps including serrated lesions, are important factors associated with interval cancers. Differences in endoscopic appearance and detection of proximal versus distal colorectal neoplasms may explain variation in the effectiveness of colonoscopy for CRC prevention65. Colonoscopy is better at detecting large and polypoid lesions whereas serrated polyps tend to be more subtle. Available evidence suggests that serrated neoplasms, particularly proximal SSA/Ps, may be important contributors to the reduced efficacy of colonoscopy for the prevention of proximal bowel cancers and for interval cancers.
Typical hyperplastic polyps are usually small (<5 mm), slightly raised, and occur most frequently in the rectosigmoid colon31. These polyps tend to flatten and are difficult to visualize when the lumen is fully distended. Differentiating hyperplastic polyps from adenomas using white-light endoscopy can be challenging and narrow band imaging may help with this distinction66. Diminutive hyperplastic polyps in the rectosigmoid colon have a negligible (if any) potential for malignancy. In contrast, SSA/Ps are usually larger than adenomas with 50% being >10 mm in size and frequently located in the proximal colon67. SSA/Ps are endoscopically subtle lesions, often flat to sessile with indistinct edges and similar color as the surrounding mucosa. The surface is soft, smooth-appearing, pale and with minimal vascularity. Adherent mucus covering the polyp is frequent and can appear yellow, green, or even rust-colored21, 25 (Figure 3A and B). Removing the adherent mucus cap can be difficult but reveals the underlying lesion (Figure 3C–F). When visualized with narrow-band imaging they appear red in color (Figure 4A and B). In contrast, TSAs typically occur in the distal colon and rectum and tend to be pedunculated or broad-based 68 making endoscopic detection less difficult.
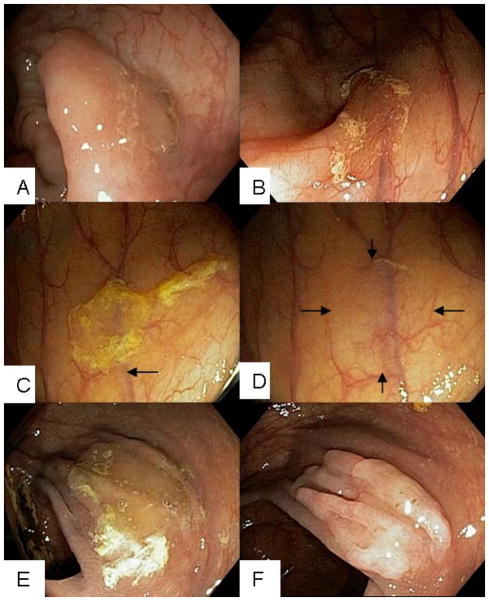
Figure 3.
Endoscopic photographs demonstrating the varied morphologic appearances of SSA/Ps. A, SSA/P with indistinct edges which overlies a mucosal fold that alters its contour. B, SSA/P in the ascending colon with a rim of debris. The lesion is shown to obscure the course of a submucosal vessel (arrow). C, SSA/P at the hepatic flexure is covered by a debris-stained mucus cap which has the characteristic “egg-drop soup” appearance. D, Lesion in C is shown after washing off the mucus cap. The SSA/P displays a subtle appearance with blurring of submucosal vessel and mucosal irregularity. E, Large SSA/P is obscured by mucus debris. F, Lesion in E after washing and aspiration of intraluminal air displays more conspicuous nodularity.
Figure 4.
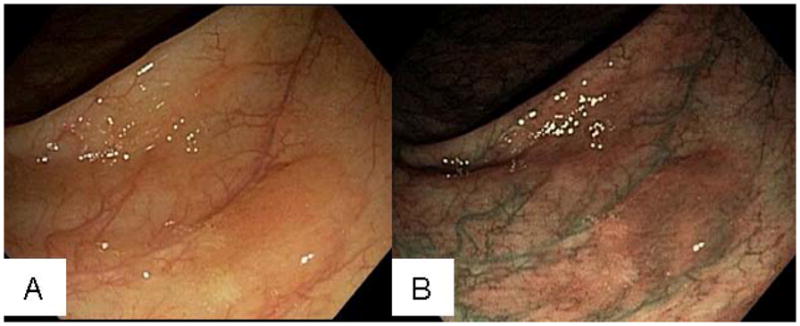
Endoscopic photographs of SSA/P. A, SSA/P in ascending colon with an adherent mucus cap in white light. B, Lesion in A under narrow-band imaging.
In an analysis of 158 SSA/Ps69 observed during routine colonoscopy, the following visual descriptors were most prevalent for SSA/Ps: mucus cap, rim of debris or bubbles (Figure 3C), alteration of the contour of a fold (Figure 3A and F), and interruption of the underlying mucosal vascular pattern69. The mucus cap was the most prevalent endoscopic feature seen in 64% of lesions69 with an “egg-drop soup” appearance (Figure 3C). By Paris Classification70, 98% of serrated lesions were flat and the vast majority of SSA/Ps were minimally elevated (Paris Class 0-IIa). A rim of debris, mucus cap, and obscuration of the vasculature were more common with SSA/Ps whereas conventional adenomas were more likely to be nodular, red, and dome-shaped69. The subtle and varied endoscopic features of SSA/Ps make them inconspicuous compared to pedunculated and/or protruding polyps and are, therefore, easily overlooked33, 71.
Detection of proximal serrated polyps during screening colonoscopy has been shown to be operator dependent and to correlate with adenoma detection rates33,71. In fact, a major factor in the detection of SSA/Ps is the diligence and experience of the endoscopist71. Some endoscopists overlook >half of serrated lesions in the proximal colon33, 71. Burnett-Hartman et al conducted a case-control study72 showing that endoscopy protected from advanced adenomas, but no statistically significant association was found between previous endoscopy and SSA/Ps72. Together, these data suggest failure to detect SSA/Ps is a likely contributor to the lower level of protection afforded by colonoscopy for the prevention of proximal vs distal colon cancers73. The extent to which detecting CRCs associated with SSA/Ps is related to rate of progression to malignancy is unknown. Regardless, increased familiarity with the subtle endoscopic appearances of SSA/Ps will likely improve detection and may improve the ability of colonoscopy to prevent CRC.
To optimize the colonoscopic detection of sessile serrated polyps, strict adherence to the following are required: a high-quality bowel preparation, adequate luminal distention at colonoscopy with careful and complete mucosal inspection, diligent washing to remove debris, and slow colonoscopic withdrawal74, 75. Chromoendoscopy is an adjunctive technique that has the potential to improve detection of serrated polyps in the proximal colon31. Chromoendoscopy uses a contrast agent, frequently indigo carmine, that is sprayed on the colonic mucosa during endoscopy and accumulates in colonic pits and grooves, thereby, highlighting flat lesions and aiding in differentiating non-neoplastic from neoplastic polyps. To enhance the endoscopic recognition of serrated polyps, Kimura et al76 evaluated magnifying chromoendoscopy that identified a novel pit pattern, referred to as Type II-open (Type II-O). This pit pattern was only moderately sensitive (65%) but highly specific (97%) for identifying SSA/P. Type II-O pits are similar to Type II pits in the Kudo classification of pit patterns77, but are wider and rounder in shape. Since the main issue is one of identifying SSA/Ps, the limited sensitivity of pit pattern analysis limits the clinical utility of this approach. The role of chromoendoscopy and other adjunctive imaging methods, including narrow-band imaging and autofluorescence for improving the detection of serrated polyps, await further study. Interestingly, a recent study showed that stool DNA testing using methylated vimentin and mutated BRAF genes was able to detect SSA/Ps, suggesting that this noninvasive technology may have a role in the detection of serrated lesions78.
Surveillance
As for conventional adenomas, CRC risk in patients with serrated polyps is dependent upon polyp size, number, and pathologic features. In contrast to conventional adenomas, however, the anatomic distribution of serrated polyps is more strongly associated with CRC risk. Specifically, large (> 1 cm) serrated polyps were the strongest predictor of CRC, particularly for proximal CRCs, in a study of 10,199 subjects in Japan undergoing their first colonoscopy59. These and other data indicate that risk stratification for serrated polyps can be performed using polyp size and location, as well as the presence or absence of dysplasia. To date, however, limited post-polypectomy longitudinal data exist upon which to base surveillance intervals after removal of serrated polyps, and current recommendations for post-polypectomy surveillance of serrated lesions are based on expert opinion9, 79, 80.
Recommendations for serrated polyps have been incorporated into recent guidelines for colonoscopy surveillance after screening and polypectomy in the United States (Table 2)81. The typical, small (<10mm), hyperplastic polyps of the rectosigmoid are very low risk lesions and do not require intensification of colonoscopy surveillance (interval 10 years). Non-dysplastic SSA/Ps less than 1 cm in size can be surveyed at 5-year intervals. Serrated polyps with any of the following three features require surveillance colonoscopy in 3 years: size ≥10mm, presence of cytological dysplasia, or traditional serrated adenoma. After piecemeal resection of a SSA, individuals should have a repeat colonoscopy in 3–6 months to evaluate the polypectomy site for residual or recurrent polyp as per conventional adenoma guidelines to ensure complete resection. Surveillance intervals may require modification based on age, family or personal history of CRC, and comorbidities. A limitation of these surveillance recommendations is underscored by patients with SSA/P and cytologic dysplasia, where a 3 year colonoscopic surveillance interval has been recommended. The appropriateness of this interval can be questioned given that SSA/Ps with cytologic dysplasia show frequent MSI and have the potential to rapidly progress to carcinoma53, 57. Therefore, managing SSA/Ps with dysplasia in a manner similar those with advanced adenomas with repeat colonoscopy at one year after initial complete resection would be reasonable at this time56. Current surveillance guidelines for serrated polyps will undoubtedly be updated as higher quality evidence becomes available.
Table 2.
Surveillance Guidelines for Serrated Polypsa
| Lesion found | Surveillance interval (yrs) |
|---|---|
| Serrated polyposis | 1 |
| Sessile serrated polyp ≥ 10 mm | 3 |
| Sessile serrated polyp with cytological dysplasia | 3 |
| Traditional serrated adenoma | 3 |
| Sessile serrated polyp(s) <10 mm with no dysplasia | 5 |
| Small (<10mm) hyperplastic polyps in rectosigmoid | 10 |
See Lieberman et al.81
The highest risk group is patients who meet criteria for the serrated polyposis syndrome whereby colonoscopy should be performed yearly with the intent to remove all proximally located serrated lesions or all serrated polyps ≥5 mm in size if there are numerous diminutive lesions. If endoscopic control of serrated polyposis is not feasible, then surgery is indicated for high polyp burden. The most common surgical procedures are extended right hemicolectomy or subtotal colectomy. Annual endoscopic surveillance of the residual colon and/or rectum is indicated.
Treatment
All colorectal polyps, with the exception of small hyperplastic-appearing polyps in the rectosigmoid, should be completely removed. Incomplete polypectomy and/or missed lesions likely account for a large percentage of interval cancers. Incomplete polypectomy and lack of adherence to follow-up surveillance were shown to be strongly associated with colorectal cancer risk after colonoscopic polyp detection in a community setting82. The issue of complete polypectomy is particularly important for SSA/Ps since frequent positive margins indicates their incomplete removal83. To ensure complete polyp excision, snare technique is recommended for polyps larger than 3 mm in size. Attempting to remove polyps greater than 4 mm in size with multiple biopsies should be avoided, as it is an ineffective polyp removal technique. When removing large sessile polyps, cauterization of the perimeter of the polypectomy site can decrease the rate of incomplete resection. If repeat attempts at colonoscopic polypectomy fail, surgical resection should be considered.
VIII. Conclusion
Recognition of the serrated neoplasia pathway represents an important advance in understanding CRC carcinogenesis with opportunities for prevention. Serrated colorectal polyps are clinically and molecularly diverse lesions that share common crypt luminal morphology characterized by glandular serration. Evidence indicates that subtypes of serrated polyps, particularly TSA, SSA/P, and SSA/P with dysplasia, may progress to adenocarcinoma through a serrated neoplasia pathway. Furthermore, data suggest that SSA/Ps are precursors of MSI colon cancers and may undergo more rapid progression to malignancy. SSA/Ps and MSI-H colon cancers are more common in the proximal colon and colonoscopy has been shown to be relatively ineffective in preventing proximal colon cancers, potentially due to missed serrated polyps. The malignant potential of the serrated neoplasia pathway is acknowledged by the inclusion of serrated polyps in recent colonoscopy surveillance guidelines. A critical step to decreasing CRC incidence via the serrated pathway is to improve detection of serrated lesions and to ensure their complete removal at endoscopy.
Abbreviations used
- CIMP
CpG island-methylator phenotype
- CRC
Colorectal carcinoma
- MMR
mismatch repair
- MSI
microsatellite instability
- SPS
serrated polyposis syndrome
- SSA/P
sessile serrated adenoma/polyp
- TSA
traditional serrated adenoma
- WHO
World Health Organization
Footnotes
Conflicts of Interest: The authors disclose no conflicts.
Author involvement with manuscript: Seth Sweetser – Design, acquisition of data, and drafting of manuscript; Thomas C. Smyrk– acquisition of data, and drafting of manuscript; Frank A. Sinicrope – drafting and critical revision of the manuscript for important intellectual content.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Huang CS, Farraye FA, Yang S, et al. The clinical significance of serrated polyps. Am J Gastroenterol. 2011;106:229–240. doi: 10.1038/ajg.2010.429. [DOI] [PubMed] [Google Scholar]
- 3.Torlakovic E, Skovlund E, Snover DC, et al. Morphologic reappraisal of serrated colorectal polyps. Am J Clin Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Ahnen DC, Burt DR, et al. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. 4. Lyon: IARC; 2010. [Google Scholar]
- 5.Yang S, Farraye FA, Mack C, et al. BRAF and KRAS mutations in hyperplastic polyps and serrated adenomas of the colorectum: relationship to histology and CpG island methylation status. Am J Surg Pathol. 2004;28:1452–1459. doi: 10.1097/01.pas.0000141404.56839.6a. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein NS, Bhanot P, Odish E, et al. Hyperplastic-like colon polyps that preceded microsatellite-unstable adenocarcinomas. Am J Clin Pathol. 2003;119:778–796. doi: 10.1309/DRFQ-0WFU-F1G1-3CTK. [DOI] [PubMed] [Google Scholar]
- 7.Li SC, Burgart L. Histopathology of serrated adenoma, its variants, and differentiation from conventional adenomatous and hyperplastic polyps. Arch Pathol Lab Med. 2007;131:440–445. doi: 10.5858/2007-131-440-HOSAIV. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J Natl Cancer Inst. 2001;93:1307–13. doi: 10.1093/jnci/93.17.1307. [DOI] [PubMed] [Google Scholar]
- 9.Snover DC, Jass JR, Fenoglio-Preiser C, et al. Serrated polyps of the large intestine. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 10.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088–100. doi: 10.1053/j.gastro.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 11.Yantiss RK, Oh KY, Chen YT, et al. Filiform serrated adenomas: a clinicopathologic and immunophenotypic study of 18 cases. Am J Surg Pathol. 2007;31:1238–45. doi: 10.1097/PAS.0b013e31802d74c0. [DOI] [PubMed] [Google Scholar]
- 12.Haramis A-PG, Begthel H, van den Born M, et al. De Novo Crypt Formation and Juvenile Polyposis on BMP Inhibition in Mouse Intestine. Science. 2004;303:1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 13.Fu B, Yachida S, Morgan R, et al. Clinicopathologic and genetic characterization of traditional serrated adenomas of the colon. Am J Clin Pathol. 2012;138:356–66. doi: 10.1309/AJCPVT7LC4CRPZSK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toyota M, Ahuja N, Ohe-Toyota M, et al. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci U S A. 1999;96:8681–6. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ogino S, Nosho K, Irahara N, et al. Prognostic significance and molecular associations of 18q loss of heterozygosity: a cohort study of microsatellite stable colorectal cancers. J Clin Oncol. 2009;27:4591–8. doi: 10.1200/JCO.2009.22.8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rajagopalan H, Bardelli A, Lengauer C, et al. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 17.Samowitz WS, Albertsen H, Herrick J, et al. Evaluation of a large, population-based sample supports a CpG island methylator phenotype in colon cancer. Gastroenterology. 2005;129:837–45. doi: 10.1053/j.gastro.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 18.Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet. 2006;38:787–93. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 19.Domingo E, Laiho P, Ollikainen M, et al. BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet. 2004;41:664–8. doi: 10.1136/jmg.2004.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arain MA, Sawhney M, Sheikh S, et al. CIMP status of interval colon cancers: another piece to the puzzle. Am J Gastroenterol. 2010;105:1189–95. doi: 10.1038/ajg.2009.699. [DOI] [PubMed] [Google Scholar]
- 21.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Prac Oncol. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 22.Fernando WWD, Whitehall VL, Leggett BA, Spring KJ. SLC5A8 methlyation, CIMP and BRAF mutation in serrated polyps of the colorectum. J Gastroenterol Hepatol. 2008;23:A218. [Google Scholar]
- 23.Mäkinen MJ, George SMC, Jernvall P, et al. Colorectal carcinoma associated with serrated adenoma – prevalence, histological features, and prognosis. J Pathol. 2001;193:286–294. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH800>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Beach R, Chan AO-O, Wu T-T, et al. BRAF mutations in aberrant crypt foci and hyperplastic polyposis. Am J Pathol. 2005;166:1069–1075. doi: 10.1016/S0002-9440(10)62327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara T, Simms LA, Whitehall VL, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–44. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whitehall VLJ, Walsh MD, Young J, et al. Methylation of O–6-Methylguanine DNA Methyltransferase Characterizes a Subset of Colorectal Cancer with Low-level DNA Microsatellite Instability. Cancer Research. 2001;61:827–830. [PubMed] [Google Scholar]
- 27.Ogino S, Kawasaki T, Ogawa A, et al. TGFBR2 mutation is correlated with CpG island methylator phenotype in microsatellite instability-high colorectal cancer. Hum Pathol. 2007;38:614–20. doi: 10.1016/j.humpath.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Yamagata S, Muto T, Uchida Y, et al. Polypoid growth and K-ras codon 12 mutation in coiorectal cancer. Cancer. 1995;75:953–957. doi: 10.1002/1097-0142(19950215)75:4<953::aid-cncr2820750409>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 29.Torlakovic EE, Gomez JD, Driman DK, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA) Am J Clin Pathol. 2008;32:21–29. doi: 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- 30.Carr NJ, Mahajan H, Tan KL, et al. Serrated and non-serrated polyps of the colorectum: their prevalence in an unselected case series and correlation of BRAF mutation analysis with the diagnosis of sessile serrated adenoma. J Clin Pathol. 2009;62:516–518. doi: 10.1136/jcp.2008.061960. [DOI] [PubMed] [Google Scholar]
- 31.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–7. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–686. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 33.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the Detection of Serrated Polyps in an Average Risk Colorectal Cancer Screening Cohort. Am J Gastroenterol. 2010;105:2656–2664. doi: 10.1038/ajg.2010.315. [DOI] [PubMed] [Google Scholar]
- 34.Kahi CJ, Li X, Eckert GJ, et al. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2012;75:515–520. [Google Scholar]
- 35.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas: a distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev. 2009;18:2310–2317. doi: 10.1158/1055-9965.EPI-09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limsui D, Vierkant RA, Tillmans LS, et al. Cigarette smoking and colorectal cancer risk by molecularly defined subtypes. J Natl Cancer Inst. 2010;102:1012–22. doi: 10.1093/jnci/djq201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeevaratnam P, Cottier DS, Browett PJ, et al. Famlial giant hyperplastic polyposis predisposing to colorectal cancer: a new hereditary bowel cancer syndrome. J Pathol. 1996;179:20–25. doi: 10.1002/(SICI)1096-9896(199605)179:1<20::AID-PATH538>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 39.Boparai KS, Reitsma JB, Lemmens V, et al. Increased colorectal cancer risk in first-degree relatives of patients with hyperplastic polyposis syndrome. Gut. 2010;59:1222–1225. doi: 10.1136/gut.2009.200741. [DOI] [PubMed] [Google Scholar]
- 40.Kalady MF, Jarrar A, Leach B, et al. Defining Phenotypes and Cancer Risk in Hyperplastic Polyposis Syndrome. Dis Colon Rectum. 2011;54:164–170. doi: 10.1007/DCR.0b013e3181fd4c15. [DOI] [PubMed] [Google Scholar]
- 41.Chow E, Lipton L, Lynch E, et al. Hyperplastic Polyposis Syndrome: Phenotypic Presentations and the Role of MBD4 and MYH. Gastroenterology. 2006;131:30–39. doi: 10.1053/j.gastro.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 42.Rubio CASS, Jaramillo E, Lindblom A. Hyperplastic polyposis coli syndrome and colorectal carcinoma. Endoscopy. 2006;38:266–70. doi: 10.1055/s-2006-925026. [DOI] [PubMed] [Google Scholar]
- 43.Rashid A, Houlihan PS, Booker S, et al. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology. 2000;119:323–32. doi: 10.1053/gast.2000.9361. [DOI] [PubMed] [Google Scholar]
- 44.Vemulapalli KCRD. Failure to recognize serrated polyposis syndrome in a cohort with large sessile colorectal polyps. Gastrointest Endosc. 2012 Jun;75:1206–10. doi: 10.1016/j.gie.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan D, Sweet K, Drini M, et al. Phenotypic diversity in patients with multiple serrated polyps: a genetics clinic study. Int J Colorectal Dis. 2010;25:703–712. doi: 10.1007/s00384-010-0907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edelstein DL, Axilbund JE, Hylind LM, et al. Serrated polyposis: rapid and relentless development of colorectal neoplasia. Gut. 2012 doi: 10.1136/gutjnl-2011-300514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boparai KS, Dekker E, Polak MM, et al. A serrated colorectal cancer pathway predominates over the classic WNT pathway in patients with hyperplastic polyposis syndrome. Am J Pathol. 2011;178:2700–2707. doi: 10.1016/j.ajpath.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lage P, Cravo M, Sousa R, et al. Management of Portuguese Patients with Hyperplastic Polyposis and Screening of At-Risk First-Degree Relatives: A Contribution for Future Guidelines Based on a Clinical Study. Am J Gastroenterol. 2004;99:1779–1784. doi: 10.1111/j.1572-0241.2004.30178.x. [DOI] [PubMed] [Google Scholar]
- 49.Win A, Walters R, Buchanan D, et al. A study of cancer risks in relatives of patients with serrated polyposis. Hered Cancer Clin Pract. 2012;10:A21. doi: 10.1038/ajg.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan AO-O, Issa J-PJ, Morris JS, et al. Concordant CpGi island methylation in hyperplastic polyposis. Am J Pathol. 2002;160:529–536. doi: 10.1016/S0002-9440(10)64872-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boparai KS, Dekker E, van Eeden S, et al. Hyperplastic Polyps and Sessile Serrated Adenomas as a Phenotypic Expression of MYH-Associated Polyposis. Gastroenterology. 2008;135:2014–2018. doi: 10.1053/j.gastro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 52.Jass JR. Hyperplastic polyps of the colorectum-innocent or guilty? Dis Colon Rectum. 2001;44:163–6. doi: 10.1007/BF02234287. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein NS. Small colonic microsatellite unstable adenocarcinomas and high-grade epithelial dysplasias in sessile serrated adenoma polypectomy specimens: a study of eight cases. Am J Clin Pathol. 2006;125:132–45. [PubMed] [Google Scholar]
- 54.Lazarus R, Junttila OE, Karttunen TJ, et al. The risk of metachronous neoplasia in patients with serrated adenoma. Am J Clin Pathol. 2005;123:349–359. doi: 10.1309/VBAG-V3BR-96N2-EQTR. [DOI] [PubMed] [Google Scholar]
- 55.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17:3208–15. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1–10. doi: 10.1016/j.humpath.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Sheridan TB, Fenton H, Lewin MR, et al. Sessile serrated adenomas with low- and high-grade dysplasia and early carcinomas. Am J Clin Pathol. 2006;126:564–571. doi: 10.1309/C7JE8BVL8420V5VT. [DOI] [PubMed] [Google Scholar]
- 58.Sawhney MS, Farrar WD, Gudiseva S, et al. Microsatellite instability in interval colon cancers. Gastroenterology. 2006;131:1700–5. doi: 10.1053/j.gastro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 59.Hiraoka S, Kato J, Fujiki S, et al. The Presence of Large Serrated Polyps Increases Risk for Colorectal Cancer. Gastroenterology. 2010;139:1503–1510.e3. doi: 10.1053/j.gastro.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 60.East JE, Saunders BP, Jass JR. Sporadic and syndromic hyperplastic polyps and serrated adenomas of the colon: classification, molecular genetics, natural history, and clinical management. Gastroenterol Clin North Am. 2008;37:25–46. v. doi: 10.1016/j.gtc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 61.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 62.Singh H, Nugent Z, Mahmud SM, et al. Predictors of Colorectal Cancer After Negative Colonoscopy: A Population-Based Study. Am J Gastroenterol. 2010;105:663–673. doi: 10.1038/ajg.2009.650. [DOI] [PubMed] [Google Scholar]
- 63.Brenner H, Hoffmeister M, Arndt V, et al. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2010;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 64.Farrar WD, Sawhney MS, Nelson DB, et al. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259–1264. doi: 10.1016/j.cgh.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 65.Rondagh EJA, Bouwens MWE, Riedl RG, et al. Endoscopic appearance of proximal colorectal neoplasms and potential implications for colonoscopy in cancer prevention. Gastrointestinal Endoscopy. 2012;75:1218–1225. doi: 10.1016/j.gie.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 66.Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572–576. doi: 10.1016/j.gie.2010.03.1124. [DOI] [PubMed] [Google Scholar]
- 67.Vu HT, Lopez R, Bennett A, et al. Individuals with sessile serrated polyps express an aggressive colorectal phenotype. Dis Colon Rectum. 2011;54:1216–1223. doi: 10.1097/DCR.0b013e318228f8a9. [DOI] [PubMed] [Google Scholar]
- 68.Oka S, Tanaka S, Hiyama T, et al. Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc. 2004;59:213–219. doi: 10.1016/s0016-5107(03)02693-2. [DOI] [PubMed] [Google Scholar]
- 69.Tadepalli US, Feihel D, Miller KM, et al. A morphologic analysis of sessile serrated polyps observed during routine colonoscopy (with video) Gastrointestinal Endoscopy. 2011;74:1360–1368. [Google Scholar]
- 70.Endoscopic Classification Review G. Update on the Paris Classification of Superficial Neoplastic Lesions in the Digestive Tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 71.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 72.Burnett-Hartman AN, Newcomb PA, Phipps AI, et al. Colorectal Endoscopy, Advanced Adenomas, and Sessile Serrated Polyps: Implications for Proximal Colon Cancer. Am J Gastroenterol. 2012;107:1213–1219. doi: 10.1038/ajg.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rex DK, Hewett DG, Snover DC. Editorial: Detection Targets for Colonoscopy: From Variable Detection to Validation. Am J Gastroenterol. 2010;105:2665–2669. doi: 10.1038/ajg.2010.330. [DOI] [PubMed] [Google Scholar]
- 74.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic Withdrawal Times and Adenoma Detection during Screening Colonoscopy. New Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 75.East JE, Bassett P, Arebi N, et al. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest Endosc. 2011;73:456–463. doi: 10.1016/j.gie.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 76.Kimura T, Yamamoto E, Yamano H-o, et al. A Novel Pit Pattern Identifies the Precursor of Colorectal Cancer Derived From Sessile Serrated Adenoma. Am J Gastroenterol. 2012;107:460–469. doi: 10.1038/ajg.2011.457. [DOI] [PubMed] [Google Scholar]
- 77.Kudo S-e, Tamura S, Nakajima T, et al. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 78.Hussain FTN, Yab TC, Harrington JJ, et al. 743 Noninvasive Detection of Serrated Colorectal Polyps by Stool Assay of Methylated Vimentin and Mutant BRAF Genes. Gastroenterology. 2010;138:S–102. [Google Scholar]
- 79.Rex DK, Ahnen DJ, Baron JA, et al. Serrated Lesions of the Colorectum: Review and Recommendations From an Expert Panel. Am J Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Terdiman JP, McQuaid KR. Surveillance Guidelines Should Be Updated to Recognize the Importance of Serrated Polyps. Gastroenterology. 2010;139:1444–1447. doi: 10.1053/j.gastro.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 81.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 82.Brenner H, Chang-Claude J, Jansen L, et al. Role of colonoscopy and polyp characteristics in colorectal cancer after colonoscopic polyp detection: a population-based case–control study. Ann Intern Med. 2012;157:225–232. doi: 10.7326/0003-4819-157-4-201208210-00002. [DOI] [PubMed] [Google Scholar]
- 83.Chandra ASA, Cerar A, Talbot IC. Clinico-pathological aspects of colorectal serrated adenomas. World J Gastroenterol. 2006;12:2770–2. doi: 10.3748/wjg.v12.i17.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]