Abstract
Research in therapeutics of neuropsychiatric disorders is increasingly focusing on drugs with new mechanisms of action, and such agents are often assessed in preclinical studies using nonhuman primates. However, researchers lack a standardized method to compare distinct drugs for common adverse effects on the nervous system. We have developed a new scale for this purpose, named “Drug Effects on the Nervous System” (DENS), and here tested its utility in an analysis of the second-generation antipsychotic risperidone in monkeys. Behavioral effects of risperidone over a ten-fold clinically relevant exposure range were rated with the DENS scale and compared with a standard motor disability scale for primates. Ratings were correlated with projected D2 and 5-HT2A receptor occupancy over time. The DENS scale detected dose-dependent side effects of risperidone involving various functions of the nervous system (cognitive, sensorimotor and autonomic functions) and, thus, beyond the motor effects detected with the motor disability scale. A consistent temporal association of DENS scale changes with projected D2 receptor occupancy was observed, and DENS scale ratings demonstrated high inter-rater reliability. These results demonstrate the usefulness of the DENS scale as a highly sensitive, reliable and accurate method to identify common adverse effects of risperidone and potentially other neurotropics for translational studies in primates.
Keywords: Neurotropics, Side-effects, Rating scale, Receptor occupancy, Normal monkeys
1. Introduction
It is widely recognized that there is a need for mechanistically novel drugs to treat neuropsychiatric disorders (Fibiger, 2012; Hyman, 2012), and the assessment of drug candidates typically depends on preclinical tests in nonhuman primates. Translational studies in primates may be used to evaluate the safety and side-effect profile of exploratory molecules prior to their Phase 1 clinical trials (Kola and Landis, 2004). Drugs with new mechanisms of action may be expected to have unique adverse effects on the nervous system, from sedation or excitation to various cognitive, motor and autonomic symptoms. However, the side-effect assessment of neurotropic drugs has been inconsistent in primate studies due to the lack of standardized measures for this purpose. With the exception of extrapyramidal effects such as those produced by dopamine D2 receptor antagonists that can be assessed efficiently with the available scales for primate models of Parkinson’s disease (Papa et al., 2004; Papa and Chase, 1996), other effects of these agents on the nervous system are usually not quantified. Therefore, the development of new neurotropic drugs typically fails to achieve a clear understanding and full assessment of adverse effects in the preclinical phases.
We have developed a scale to measure behavioral “Drug Effects on the Nervous System” (DENS) in nonhuman primates based on the most common side effects of neurotropics (i.e.: drugs with effects on the CNS that are used for a variety of neurologic and psychiatric disorders) that can be readily assessed in the monkey. To test the usefulness of this new scale, we assessed the behavioral effects of the second-generation antipsychotic risperidone (Risperdal®) across a clinically relevant ten-fold exposure range and with temporal correlation to projected D2 and 5-HT2A receptor occupancy. Classic antipsychotics have significant side effects on the cognitive and sensorimotor systems in patients (Day et al., 1995; Lingjaerde et al., 1987), and recent studies have reported that atypical antipsychotics can also produce the same motor side effects, i.e.: the development of parkinsonian motor deficits and dyskinesias (Casey, 1996; Stip, 2000). Risperidone was chosen for this analysis because its profile of motor and non-motor behavioral effects is well documented in humans (De Deyn et al., 1999). As expected, risperidone produced a number of effects that were captured by the DENS scale ratings. A standard motor disability scale was also used to assess risperidone effects, and the comparison of measurements between scales allowed us to determine the sensitivity of the DENS scale. Altogether, results demonstrated that the newly developed scale DENS is an accurate and sensitive tool to evaluate typical side effects of risperidone in primates, and the newly described scale can now be further tested with a variety of other neurotropic agents.
2. Materials and Methods
2.1. Pharmacokinetic Studies and Projection of Receptor Occupancy
Pharmacokinetics
Studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, 1996) using a protocol reviewed and approved by the Pfizer Worldwide Research and Development Institutional Animal Care and Use Committee (Groton, CT). Single doses (0.01 or 0.1 mg/kg, s.c.) of risperidone (Sigma-Aldrich, St. Louis, MO) in 0.1% 1 N HCl in saline (pH 5) were administered (0.1 ml/kg) to macaques (Macaca fascicularis, N=1/gender/dose, 7 to 9 kg). Blood samples were obtained via a vascular access port just before dosing and at 0.25, 0.5, 0.75, 1, 1.5, 2, 4 and 6 h post-injection following standard procedures. Monkeys were continuously monitored for any readily apparent adverse events by trained laboratory personnel for up to 6 h following dosing. Collected plasma samples were stored at −20 °C until processing for bioanalysis.
Receptor occupancy projections
Due to the metabolism of risperidone to an active metabolite (9-hydroxyrisperidone) (Schotte et al., 1996), both risperidone and 9-hydroxyrisperidone were quantified in plasma samples using a published bioanalytical method (Doran et al., 2012). To account fully for both active molecules pharmacologically throughout the DENS assessment, the total plasma compound concentration in the monkeys (Cp) for both risperidone and 9-hydroxyrisperidone at each pharmacokinetic sampling time point were converted to a projected total receptor occupancy (RO) at both D2 and 5-HT2A receptors. This was accomplished by first converting Cp to a projected unbound brain compound concentration (Cb,u) using the respective compound’s molecular weight (MW; risperidone (411 ng/nmol), 9-hydroxyrisperidone (427 ng/nmol)), fraction unbound in plasma (fu,p; risperidone (0.0840) and 9-hydroxyrisperidone (0.185)), unbound brain compound concentration-to-unbound plasma compound concentration ratio (Cb,u:Cp,u; risperidone (0.89), 9-hydroxyrisperidone (0.19)) (Doran et al., 2012), and Eq. 1:
| (1) |
Subsequently, the fraction of D2 or 5-HT2A receptors occupied exclusively by either risperidone (FRORisp) or 9-hydroxyrisperidone (FRO9-OHRisp) were calculated using respective projected Cb,u (via Eq. 1), the reported (Schotte et al., 1996) human binding affinity (Ki) for D2 (risperidone (5.9 nM), 9-hydroxyrisperidone (4.8 nM)) or 5-HT2A (risperidone (0.52 nM), 9-hydroxyrisperidone (1.0 nM)), and Eq. 2 (Shaffer, 2010):
| (2) |
Lastly, total D2 or 5-HT2A receptor occupancy (RO) accounting for both active entities was calculated using FRORisp and FRO9-OHRisp (via Eq. 2) and Eq. 3 (Venkatakrishnan et al., 2007):
| (3) |
2.2. Behavioral Studies
Animals and risperidone tests
We used for behavioral studies 4 macaques (Macaca Mulatta), one male and three females, all adults 5 to 8 kg. Monkeys were kept in controlled housing conditions with free access to food and water. All studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals (ILAR, 1996), and approved by the Institutional Animal Care and Use Committee. The dose range of risperidone (Sigma-Aldrich) selected for the experiments (0.01, 0.032, 0.1 mg/kg) was based on previous primate studies where 0.01 mg/kg, i.m. induced measurable behavioral changes in Cebus monkeys (Casey, 1996). Risperidone was dissolved in distilled water and administered subcutaneously. The corresponding vehicle injections were used in control experiments. Animals were evaluated for baseline behavior before drug administration on each testing day. After s.c. injection (time 0), animals were evaluated starting at 30 min and continued thereafter every 20 min until either 170 min or the animal returned to its baseline scores. All tests were performed in the morning after an overnight fast, and under the same environmental conditions. Each test was repeated three times, and data were averaged to yield a mean from three data points for each risperidone dose in each monkey for statistical analysis.
Behavioral assessment
The new scale was developed to evaluate in the monkey side effects commonly induced by antipsychotics and other drugs with actions on the nervous system.
This new scale was named “Drug Effects on the Nervous System” (DENS), and is composed of three categories, namely, cortical, extrapyramidal motor and autonomic functions. Extrapyramidal function included only two items because extensive evaluation of parkinsonism can be performed using the specific scales for motor disability. The inclusion here of involuntary movements (dyskinesias) was based on their frequent occurrence even in the absence of parkinsonian disability. Eye movements can also serve as an indication of minimal extrapyramidal compromise although sedation may also affect them, and thus this item needs to be interpreted together with the scores obtained for cortical function. The individual items of the DENS scale and their detailed rating are:
-
-Cortical Function:
- Attentiveness (The animal attention to the environment was assessed as: Attentive/Active = 0, Less Attentive/Less Active = 1, Not Attentive/Inactive = 2)
- 2) Reactivity (Animal responds to touch of the cage or the animal’s body by threatening or trying to reach the examiner = 0, Animal only responds by looking at the examiner = 1, Lack of responses = 2, Animal appears sleepy, eyelids drooping, somnolence = 3)
-
-Extrapyramidal Motor Function:
- Eye Movement (Frequent blinking/normal eye movement = 0, Less frequent blinking/reduced or slow eye movement = 1, Very infrequent blinking, fixed eyes = 2)
- Involuntary movements (Absent = 0, Mild = 1, Moderate = 2, Severe = 3)
-
-Autonomic Function:
- Salivation (Normal salivation = 0, increased salivation with some dribbling = 1, Drooling = 2)
- Upper GI (gastrointestinal) tract function (Normal appetite and interests in treats and rewards = 0, Decreased interest in food, the animal needs to be highly encouraged to take rewards = 1, No interest in any rewards, the animal is nauseated or vomiting = 2)
- Lower GI tract function (No defecation or formed stool = 0, A large amount of stool or softer stool = 1, Diarrhea = 2)
- Urinary function (One or two urine spots on cage pan = 0, Frequent urination = 1, Very frequent urination = 2)
Total range of DENS score is 0 to 18.
Opposite autonomic effects on lower GI function (diminished bowel movements, chronic constipation), and urinary function (urinary retention) that can be caused by drugs with CNS effects (e.g. anticholinergic effects) were not included in this scale because they cannot be accurately assessed after acute drug injections.
The behavioral effects of risperidone were assessed with the new scale DENS and the standardized Motor Disability Scale for MPTP-treated monkeys (Papa and Chase, 1996) for comparison. The two scales were applied simultaneously in each drug test. Scoring using both scales was done by direct examination with the examiner blinded to the treatment. Animals were also videotaped for deferred scoring by another blinded examiner. Videotapes were taken in all tests and kept unlabeled for scoring. To assess the reliability of the DENS scale, the inter-rater variability was determined comparing results obtained with the two blinded examiners (Gwet, 2008). For this analysis, videotapes of tests from all animals were scored at four time points: 50, 90, 130 and 170 min. The second examiner scoring the videotapes was fully trained to evaluate behaviors of normal monkeys, and was not involved in the direct examination of the animals.
2.3. Statistical analysis
Total scores of DENS and motor disability scores (MDS) were graded within wide ranges, and scale items that were analyzed individually included non-integer values; thus, data formed continuous variables. Two-factor analysis of variance (ANOVA) for repeated measures followed by the Tukey post-hoc test was used to compare data in behavioral tests. Statistical comparisons to establish inter-rater reliability were performed using Student’s paired t-test. The significance level was set at p < 0.05. All results are expressed as mean ± S.E.M.
3. Results
3.1. Pharmacokinetics and Receptor Occupancy
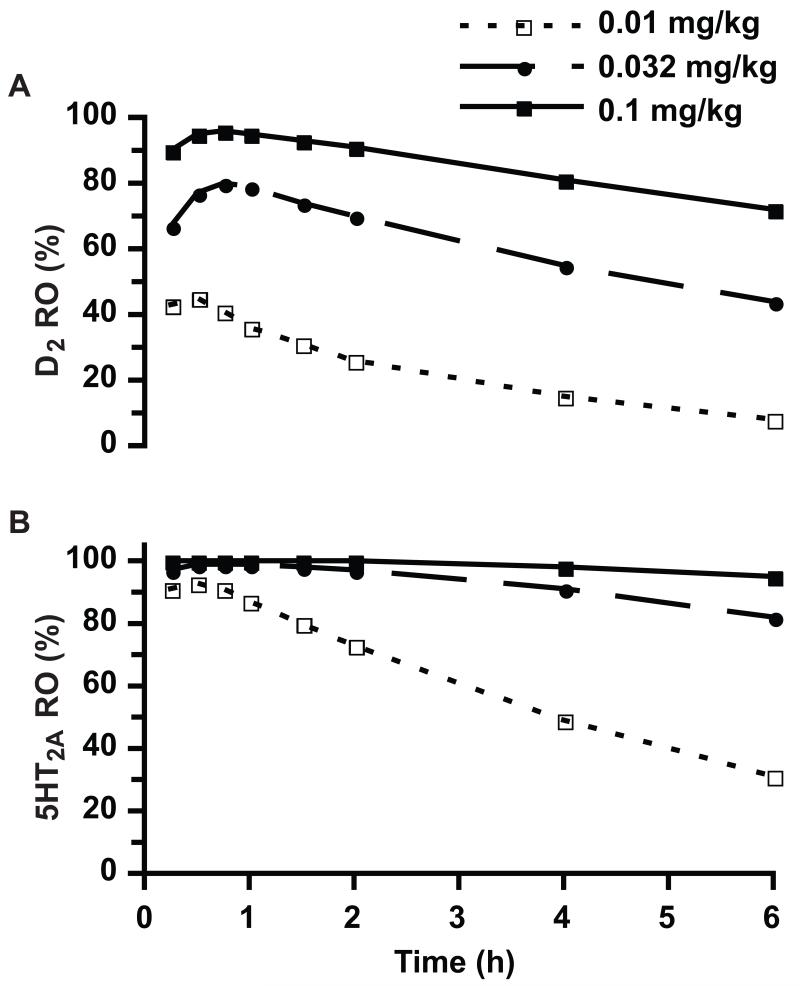
In macaques, risperidone and 9-hydroxyrisperidone exposure-time relationships were essentially linear with risperidone dose suggesting stationary pharmacokinetics and consistent compound absorption from the injection site across the behaviorally evaluated ten-fold dose range. Due to this linear exposure-dose relationship, the RO versus time plot for the 0.032 mg/kg, s.c. dose used projected active moiety Cp that were linearly extrapolated from the respective Cp (for both risperidone and 9-hydroxyrisperidone) determined from the 0.1 mg/kg, s.c. dose. Figure 1 contains both projected D2 (panel A) and 5-HT2A (panel B) RO versus time for each tested risperidone dose. To evaluate the validity of this RO projection methodology, these monkey pharmacokinetic data were used to forecast a mean D2 RO in monkeys from 0.25 to 4 h after a single 0.05 mg/kg, s.c. dose of risperidone (using the identical dose-corrected linear extrapolation methodology from the 0.1 mg/kg, s.c. Cp versus time data). At this specific dose over the cited time period, the calculated mean D2 RO was 72%, which mirrored the 75% reported (Mukherjee et al., 2001) using [18F]fallypride and positron emission tomography (PET), and this correlation provided high confidence in this RO projection methodology for understanding the risperidone-mediated exposure-RO-DENS relationship.
Figure 1.
Projected D2 (panel A) and 5-HT2A (panel B) receptor occupancy following a single subcutaneous injection of risperidone in monkeys. Data corresponding to the 0.032 mg/kg dose was generated by linear extrapolation of the pharmacokinetic data obtained with the other two doses (0.01 and 0.1 mg/kg; each tested in two monkeys). Each data point is the respective projected receptor occupancy calculated from the averaged (n=2) total plasma risperidone and 9-hydroxyrisperidone concentrations for the specific time point (0.25, 0.5, 0.75, 1, 1.5, 2, 4 and 6 h).
The projected risperidone D2 RO falls below 70% by 4 h after administration of 0.032 mg/kg s.c. in parallel with the end of behavioral effects detected with the DENS and the motor disability scales. In contrast, the projected risperidone 5-HT2A RO remains above 80% by 6 h after administration of 0.032 mg/kg s.c. when no behavioral effects were detected. The lack of prolonged motor effects at this dose of risperidone may suggest that 5HT2A receptor blockade is not involved in the motor effects of antipsychotics; however, this needs to be further examined with appropriate tests of agents with different sensitivity for the 5HT2A receptor. Furthermore, the lowest risperidone dose (0.01 mg/kg, s.c.) that had no apparent effects on the DENS and motor disability scale items was associated with projected D2 RO below 45%, but 5-HT2A RO above 70% for up to 2 h post-injection (Figure 1).
3.2. Behavioral Effects
DENS scale
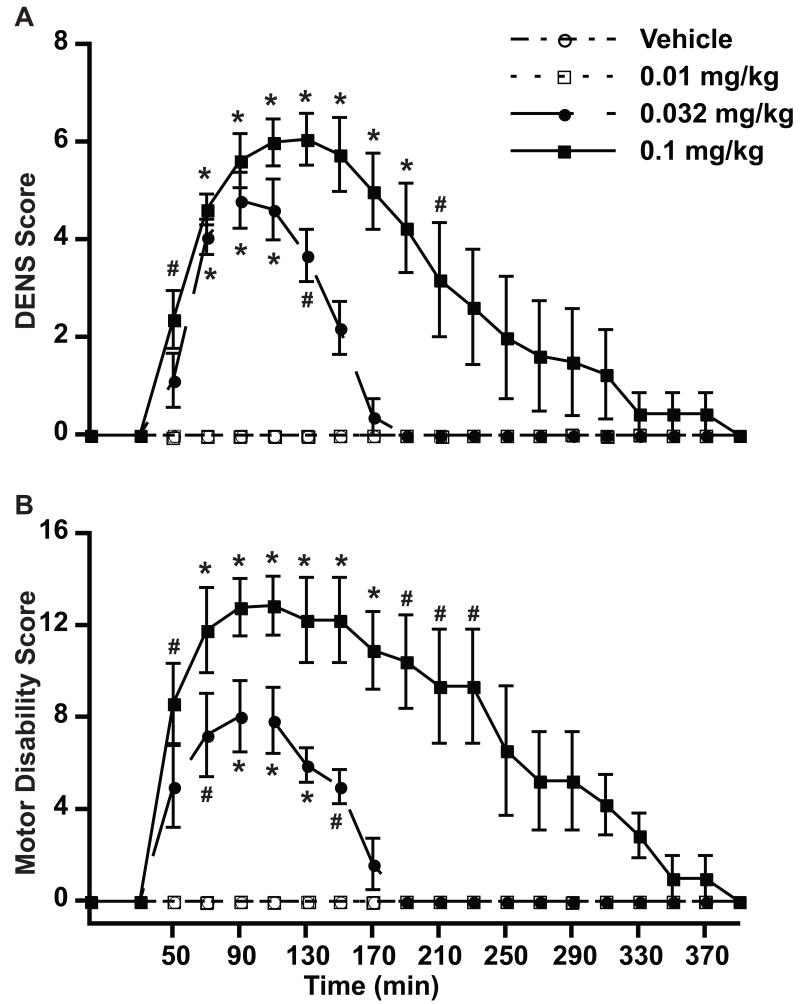
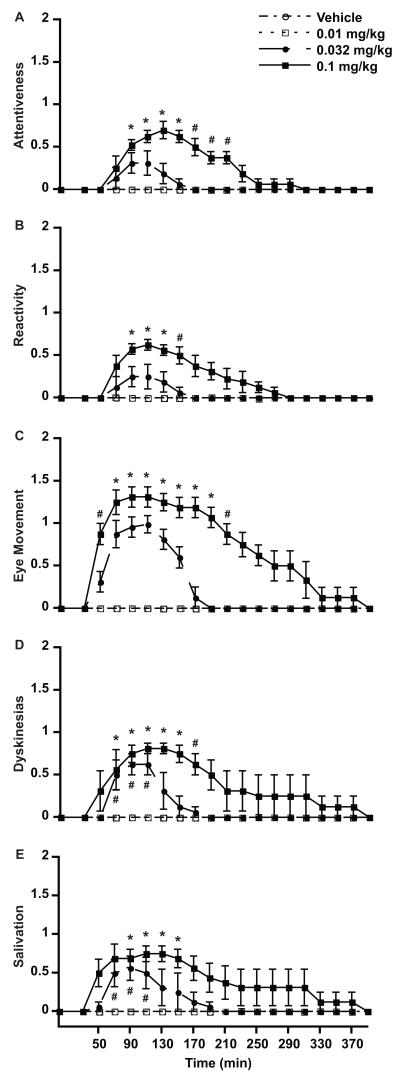
Risperidone had effects on the nervous system predominantly involving motor and autonomic functions that could be evaluated thoroughly with the new DENS scale (Figure 2A). Total scores were mainly composed of cognitive items (attentiveness and reactivity), eye movement, involuntary movements and salivation (Figure 3). Attentiveness and reactivity revealed some sedative effects of risperidone, although these effects did not dominate the monkey’s behavior. Most animals had clear extrapyramidal symptoms that were expressed in other items of the scale by parkinsonism (eye movements and salivation) and involuntary movements. Specific symptoms of parkinsonism were extensively evaluated by the motor disability scale (see below). In all monkeys, risperidone induced involuntary movements that were predominantly choreodystonic oral dyskinesias (Figure 3) as peak-dose effects disappeared 190 min after drug injection. A clear dose-response relationship was obtained with the DENS scale for total scores and individual items indicating accuracy of measurements of drug effects.
Figure 2.
Side effects induced by risperidone, as measured with the DENS scale. A. DENS scores. B. Motor Disability Scores. Drug-induced parkinsonism was measured using the standard Motor Disability Scale (MDS) for comparison of dose response curves obtained with the DENS scale. The baseline total score was taken just before drug injection (time 0). After injection scoring starts at 30 min and continues thereafter every 20 min. Values are the average of “total scores” for individual intervals from all monkeys. * p < 0.01, # p < 0.05 vs. same time point of the control test, vehicle injection. ANOVA for repeated measures followed by the post-hoc Tukey test. Data points are mean (n = 4) ± SEM.
Figure 3.
Side effects identified with individual categories of the DENS scale. A. Attentiveness, B. Reactivity, C. Eye Movement, D. Involuntary movements (Dyskinesias) and E. Salivation. The baseline score was taken just before drug injection (time 0). After injection scoring starts at 30 min and continues thereafter every 20 min. Values are the average of total scores for individual intervals from all monkeys. * p < 0.01, # p < 0.05 vs. same time point of control test, vehicle injection. ANOVA for repeated measures followed by the post-hoc Tukey test. Data points are mean (n = 4) ± SEM.
Motor disability scale
The comparison of results obtained with the DENS and motor disability scales provided a correlation of measurements using a well-established scale for parkinsonian motor deficits that may be produced by varied antipsychotics including risperidone. The motor disability scale is composed of items that specifically score bradykinesia and other parkinsonian symptoms (posture, gait, mobility, hand movement, leg movement, climbing, tremor, etc.) (Papa et al., 2004; Papa and Chase, 1996) At 0.032 and 0.1 mg/kg, risperidone induced clear parkinsonian motor disability (Figure 2B). The correspondence of risperidone dose-dependent effects between parkinsonian symptoms and other CNS effects assessed in the DENS scale (including other extrapyramidal effects such as involuntary movements, salivation, etc.) provide further evidence for the accuracy of results obtained with this newly developed scale. In addition, the DENS scale identified dose-dependent sedative effects that are common problems of psychotropics.
Inter-rater reliability
The results obtained by two independent evaluators indicated high inter-rater agreement and reliability. The two independent examiners scoring the animals were blind to the treatments and had no knowledge of the scores assigned by each other. There was no significant difference in scores between raters in either test of the dually evaluated doses (i.e. 0.032 and 0.1 mg/kg, s.c.). A similar correlation of scores between the two raters was found in analysis of the total (Figure 4A) and individual item values (Figure 4B-D) of the DENS scale (p > 0.05 for differences between scores of the same time).
Figure 4.
Inter-rater reliability with the DENS scale. A. Total DENS, B. Attentiveness, C. Reactivity, and D. Dyskinesias. Comparisons between two blind raters included tests of two doses of risperidone (0.0316 mg/kg, Low Dose; 0.1 mg/kg, High Dose). Data obtained by the first examiner (Rater 1) for a given time point and dose were compared with the corresponding data obtained by the second examiner (Rater 2). Differences between the raters were not significant (p > 0.05, Paired t-test). Data points are mean (n = 4) ± SEM.
4. Discussion
The application of the DENS scale to assess the side effects of risperidone in monkeys successfully produced dose-response curves of common side effects on the nervous system and data that correlated well with a standardized primate scale for motor disability. Additionally, the inter-rater reliability of results was high, and the analysis showed score agreement between raters plus consistency within raters. The reproducibility was maintained on re-testing, and results of the same order were obtained when examiners rated a different dose and throughout time intervals. The DENS scale, thus, evidenced robust measuring properties and high inter-rater reliability. The accuracy of temporally drug-induced behavioral changes was confirmed by projected D2 RO of ≥70% resulting in DENS effects, which are primarily mediated by D2 antagonism. Therefore, the DENS scale has proven highly sensitive, reliable and accurate to detect side effects of risperidone, and these data indicate the potential of the scale to be used widely to profile new neurotropic agents preclinically in primates. Further tests of the DENS scale using other drugs and comparatives measurements are needed to validate the scale as a method for widespread application.
Risperidone (0.025–0.2 mg/kg, p.o.) has been reported to induce dose-related dystonia in primates (Casey, 1993; Kumar et al., 2003), similar to the choreodystonic dyskinesias found here. However, other side effects in monkeys have not been reported. The application of the DENS scale was useful to document such effects. Total DENS scores in these tests of risperidone derived mostly from items such as involuntary movements, eye movement, and salivation. Attentiveness and reactivity contributed the least to the total DENS scores indicating that sedation was a minor effect of risperidone at the tested doses. These results are consistent with previous reports of little sedation induced by risepridone in primates (Casey, 1996; Kumar et al., 2003). Also in agreement, clinical studies have shown that the major effects of risperidone at therapeutic doses are not attributable to sedation (De Deyn et al., 1999). In this context of minor sedative effects, the observed reduced eye/eyelid movement that can be affected by both conditions (sedation and parkinsonism), is probably expressing more of the underlying extrapyramidal compromise with risperidone. Thus, results obtained with the DENS scale clearly indicate that the tested risperidone doses produce slight sedation but significant extrapyramidal side effects. Notably, a large number of drugs, antipsychotics and other psychotropics, can produce more significant sedation underscoring the importance of items such as attentiveness and reactivity in a primate scale for common side effects on the nervous system. Chronic administration of risperidone was not tested here, and that is necessary to determine if the sensitivity of the DENS scale to detect symptoms of both cortical and extrapyramidal dysfunction could also be useful to assess the evolution of side effects over prolonged drug treatment. The DENS scale was designed with a primary focus on common, major changes in brain functions that are seen after administration of psychotropic drugs to monkeys. The important feature of this method for assessments of drug effects in monkeys is the inclusiveness of various nervous system functions while keeping simplicity in data collection, as shown by the selection of items that can be readily assessed in monkeys.
The effects of risperidone measured with both DENS and motor disability scales were observed at doses (0.032 and 0.1 mg/kg, s.c.) projected to produce D2 RO ≥70%. This is consistent with the clinical data obtained with PET imaging showing that risperidone treatment induces extrapyramidal side effects with D2 RO ≥70% (Nyberg et al., 1999). In contrast, there was essentially no effect of risperidone at the dose of 0.01 mg/kg, despite the fact that this dose was projected to produce a maximal 5-HT2A RO of 93%. This lack of effect at the low risperidone dose is consistent with clinical observations that 5-HT2A antagonists produce few overt side effects despite the fact that such agents may impact sleep quality (Landolt and Wehrle, 2009; Terry et al., 2005). Therefore, the DENS scale appears sensitive and accurate to detect both the presence and absence of side effects of neurotropics in primates.
The presented data, including the inter-rater reliability and the pharmacokinetic data, describe the DENS scale as a useful tool for drug safety studies involving nervous system functions in primates. This new scale is intended to provide a rapid and reliable method for identifying the neurological adverse effects of a new drug. However, the scale was not developed as a method to assess thoroughly specific drug effects in the monkey that may require complex tests using various paradigms or tasks. As a general and elemental tool, the DENS scale needs yet to be validated, but the present data suggest that it could potentially be extensively applied and demonstrated valuable for screening toxic neurological drug effects in primates. Similar to the value of the Mini-Mental State Examination (MMSE) (Folstein et al., 1975; Koch et al., 2005), which is used as a “screening test” for cognitive deficits in clinical studies, the DENS scale also provides a primary approach to identify drug effects that may then require complete assessment with more complex techniques in monkeys. Further experience with a variety of drug tests may lead to refinements and improvements of the newly developed DENS scale that has shown to be a sensitive and reliable method in this study.
Highlights.
We described a new scale to assess adverse drug effects on the nervous system in primates.
We compared the new scale with a classic motor scale.
Behavioral data were temporally correlated to the projected receptor occupancy.
The new scale is accurate and sensitive to assess common side effects of neurotropics.
Acknowledgments
This work was supported by NIH grant NS045962 and NS073994 (S.M.P.), NCRR P51RR000165 and ORIP/OD P51OD011132 (Yerkes National Primate Research Center), and Pfizer, Inc. We thank Jessica S. Whithear for her valuable assistance in data collection.
Abbreviations
- Cp
plasma compound concentration
- Cb.u
unbound brain compound concentration
- CNS
central nervous system
- DENS
Drug Effects on the Nervous System
- GI
gastrointestinal
- MDS
motor disability scores
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- RO
receptor occupancy
- s.c.
subcutaneous
- MMSE
Mini-Mental State Examination
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Casey DE. Behavioral effects of sertindole, risperidone, clozapine and haloperidol in Cebus monkeys. Psychopharmacology. 1996;124:134–40. doi: 10.1007/BF02245613. [DOI] [PubMed] [Google Scholar]
- Casey DE. Serotonergic and Dopaminergic Aspects of Neuroleptic-Induced Extrapyramidal Syndromes in Nonhuman-Primates. Psychopharmacology. 1993;112:S55–S9. doi: 10.1007/BF02245007. [DOI] [PubMed] [Google Scholar]
- Day JC, Wood G, Dewey M, Bentall RP. A Self-Rating Scale for Measuring Neuroleptic Side-Effects - Validation in a Group of Schizophrenic-Patients. Brit J Psychiat. 1995;166:650–3. doi: 10.1192/bjp.166.5.650. [DOI] [PubMed] [Google Scholar]
- De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PLJ, Eriksson S, Lawlor BA. A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology. 1999;53:946–55. doi: 10.1212/wnl.53.5.946. [DOI] [PubMed] [Google Scholar]
- Doran AC, Osgood SM, Mancuso JY, Shaffer CL. An evaluation of using rat-derived single-dose neuropharmacokinetic parameters to project accurately large animal unbound brain drug concentrations. Drug Metab Dispos. 2012;40:2162–73. doi: 10.1124/dmd.112.046391. [DOI] [PubMed] [Google Scholar]
- Fibiger HC. Psychiatry, The Pharmaceutical Industry, and The Road to Better Therapeutics. Schizophrenia Bull. 2012;38:649–50. doi: 10.1093/schbul/sbs073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gwet KL. Computing inter-rater reliability and its variance in the presence of high agreement. Brit J Math Stat Psy. 2008;61:29–48. doi: 10.1348/000711006X126600. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Revolution stalled. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003142. 155cm11. [DOI] [PubMed] [Google Scholar]
- ILAR . In: Guide for the Care and Use of Laboratory Animals. Institute of Laboratory Animal Resources-Commission on Life Sciences NRC, editor. Washington DC: [Google Scholar]
- Koch HJ, Gurtler K, Szecsey A. Correlation of Mini-Mental-State-Examination (MMSE), Syndrom-Kurztest (SKT) and Clock test (CT) scores in patients with cognitive impairment assessed by means of multiple regression and response surface analysis. Arch Gerontol Geriat. 2005;40:7–14. doi: 10.1016/j.archger.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3:711–5. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- Kumar R, Palit G, Dhawan BN. Comparative behavioural effects of typical and atypical antipsychotic drugs in rhesus monkey. Eur J Pharmacol. 2003;462:133–8. doi: 10.1016/s0014-2999(02)02957-6. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci. 2009;29:1795–809. doi: 10.1111/j.1460-9568.2009.06718.x. [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The Uku Side-Effect Rating-Scale - a New Comprehensive Rating-Scale for Psychotropic-Drugs and a Cross-Sectional Study of Side-Effects in Neuroleptic-Treated Patients - Preface. Acta Psychiat Scand. 1987;76:7. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee J, Christian BT, Narayanan TK, Shi B, Mantil J. Evaluation of dopamine D-2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallypride. Neuropsychopharmacology. 2001;25:476–88. doi: 10.1016/S0893-133X(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Eriksson B, Oxenstierna G, Halldin C, Farde L. Suggested minimal effective dose of risperidone based on PET-measured D-2 and 5-HT2A receptor occupancy in schizophrenic patients. Am J Psychiat. 1999;156:869–75. doi: 10.1176/ajp.156.6.869. [DOI] [PubMed] [Google Scholar]
- Papa SM, Auberson YP, Greenamyre JT. Prolongation of levodopa responses by glycine(B) antagonists in parkinsonian primates. Ann Neurol. 2004;56:723–7. doi: 10.1002/ana.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in Parkinsonian monkeys. Ann Neurol. 1996;39:574–8. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PFM, Gommeren W, Luyten WHML, VanGompel P, Lesage AS, DeLoore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: In vitro and in vivo receptor binding. Psychopharmacology. 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Shaffer CL. Defining Neuropharmacokinetic Parameters in CNS Drug Discovery to Determine Cross-Species Pharmacologic Exposure-Response Relationships. Annu Rep Med Chem. 2010;45:55–70. [Google Scholar]
- Stip E. Novel antipsychotics: issues and controversies. Typicality of atypical antipsychotics. J Psychiatr Neurosci. 2000;25:137–53. [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Bartoszyk GD. Selective serotonin 5-HT2A receptor antagonist EMD 281014 improves delayed matching performance in young and aged rhesus monkeys. Psychopharmacology. 2005;179:725–32. doi: 10.1007/s00213-004-2114-1. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan K, Tseng E, Nelson FR, Rollema H, French JL, Kaplan IV, Horner WE, Gibbs MA. Central nervous system pharmacokinetics of the mdr1 p-glycoprotein substrate CP-615,003: Intersite differences and implications for human receptor occupancy projections from cerebrospinal fluid exposures. Drug Metab Dispos. 2007;35:1341–9. doi: 10.1124/dmd.106.013953. [DOI] [PubMed] [Google Scholar]