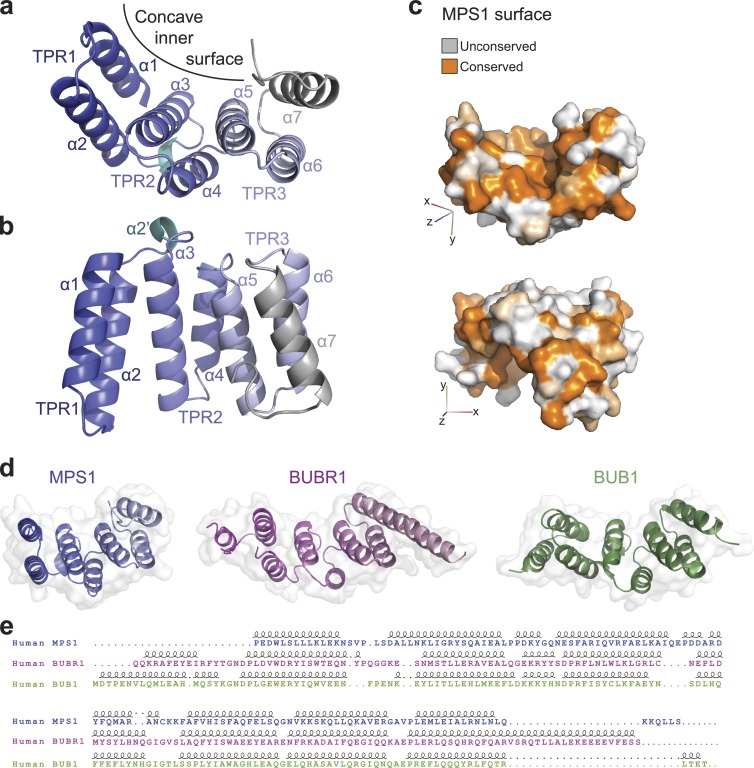
Figure 1.
Crystal structure of the MPS1 TPR domain. (a) Crystal structure of the TPR domain. A cartoon diagram of the three TPR1–3 helical doublets forming the concave surface is shown in blue shades that fade toward gray form the N toward the C terminus; the C-terminal helix is in gray, and the α2′ short helix between TPR1 and TPR2 is in cyan. (b) A side view of the TPR domain. (c) A surface representation of the TPR domain colored by sequence conservation among vertebrate MPS1 TPR domains; the top view emphasizes the conservation of the concave inner surface, and the bottom view shows some conserved patches on the generally unconserved outer surface. (d) The TPR domains of MPS1, BUBR1, and BUB1 are shown in the same orientation after structural superposition, as cartoon diagrams within a transparent surface. (e) The sequence alignment resulting from the structural superposition of the three TPR domains above is shown together with secondary structure elements. Dots indicate gaps. Loops indicate helices.