Abstract
The objective of this study was to determine the accuracy, precision, bias, and reliability of percent fat (%fat) determined by air-displacement plethysmography (ADP) with the Pediatric Option against the 4-compartment model in 31 children (4.1 ± 1.2 yr., 103.3 ± 10.2 cm., 17.5 ± 3.4 kg.). %fat was determined by (BOD POD® Body Composition System, COSMED USA, Inc., Concord, CA) with the Pediatric Option. Total body water was determined by isotope dilution (2H2O; 0.2 g/kg) while bone mineral was determined by DXA (Lunar iDXA v13.31; GE, Fairfield, CT and analyzed using enCore 2010 software). The 4-compartment model by Lohman was used as the criterion measure of %fat. The regression for %fat by ADP versus %fat by the 4-compartment model did not deviate from the line of identity where: y = 0.849(x) + 4.291. ADP explained 75.2% of the variance in %fat by the 4-compartment model while the SEE was 2.09 %fat. The Bland Altman analysis showed %fat by ADP did not exhibit any bias across the range of fatness (r = 0.04; p = 0.81). The reliability of ADP was assessed by the coefficient of variation (CV), within-subject SD, and Cronbach’s alpha. The CV was 3.5%, within-subject SD was 0.9%, and Cronbach’s alpha was 0.95. In conclusion, ADP with the Pediatric Option is accurate, precise, reliable, and without bias in estimating %fat in children 2–6 years old.
INTRODUCTION
Air-displacement plethysmography (ADP) has been shown to be a valid technique in infants (1–8 kg), children (> 25 kg), and adults for the determination of whole body composition (1–8). In 1995, the first study validating ADP (BOD POD® Body Composition System, COSMED USA, Concord, CA) in an adult population was published (7). Validation studies in children using a 4-compartment model (1) and a 3-compartment model (9) as criterion methods followed. Since then, multiple studies using chemical analysis (8), deuterium dilution (5, 10) and 4-compartment model (11) as criterion methods have found the infant ADP system (PEA POD® Infant Body Composition System, COSMED USA, Concord, CA) to be a valid technique in infants.
Currently, a gap exists in the validation of ADP in children between ≈6 months to 6 years of age. This is predominately due to the inability of infants to sit unaided and safely in the BOD POD system, particularly in infants 6 months to 3 years old where a restraint system is imperative. Additionally, no equations to estimate thoracic gas volume (TGV) in this age range exists, making it impossible to adjust body volume to compensate for isothermal properties of air trapped in the thoracic area.
Recently, a new Pediatric Option for the BOD POD GS model (COSMED USA, Concord, CA) that includes both hardware (custom designed seat and pediatric calibration cylinder see Figure 1) modifications and software changes has been developed with the purpose of testing subjects between the ages of 2 and 6 years. If ADP with the Pediatric Option interface is shown to be valid, it would allow researchers and clinicians to use the same technology to measure body composition starting in infancy and follow them into adulthood. The ability to follow infants longitudinally using the same technology starting at birth has the potential to unravel some knotty questions. For example, it has been reported that even at two weeks of age offspring from overweight/obese mothers have greater whole body fat compared to offspring from normal weight mothers, however it remains unknown if this observation persists or is a transient observation that dissipates over time, or if this increased adiposity negatively impacts future health (12, 13).
Figure 1.
Picture of the Pediatric Option seat and Calibration Cylinder.
Therefore, the purpose of this study was to examine the accuracy, precision, bias, and reliability in body fat estimates using the ADP Pediatric Option in children between 2 to 6 years of age when compared to the criterion 4-compartment model (14).
METHODS
Seventy-four infants/children enrolled into the study (i.e. both Phase I and Phase II). Subjects were screened over the telephone. Subjects had to be between 6 months to 6 years of age. The study was approved by the University of Oklahoma Health Sciences Center Institutional Review Board while written informed consent was obtained from each volunteer/parent prior to testing.
PROTOCOL
The study was divided into two phases, a β-testing phase and a validation phase. During β-testing (Phase 1), study equipment (i.e. DXA, ADP with the Pediatric Option, and total body water) were carefully scrutinized to ensure they were being employed in an appropriate manner for this population. All methods/protocols (i.e. system used to minimize movement during DXA, administration of total body water that is age appropriate, and effect of movement/crying during the ADP volume measurement) were critically analyzed.
The validation phase (Phase II) involved recruiting additional subjects. The testing sequence was as follows: Parents were instructed to arrive with their child in a semi-fasted state (i.e. no food or water for two hours). For children less than three years, parents were encouraged to feed their child as they normally would with the study team trying to test subjects during periods between meals. At no time were children deprived of food or drink if the parent deemed it hurtful to their child with the study team recording the time and amount of food given accordingly. ADP testing occurred first. Upon completion of the first trial a second trial was performed in order to obtain test re-test reliability. If the second test was valid a DXA test was then performed. At this time, if ADP and DXA tests were valid then a baseline urine sample was collected and the child was dosed with D20 (see description of dosing in the methods section). Approximately three days later the parent returned with the child’s three day urine samples.
Assessment of %fat by air-displacement plethysmography (ADP)
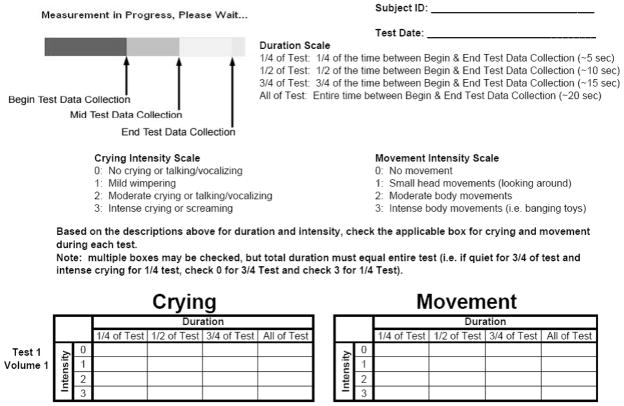
Total body volume and density along with %fat was evaluated with air-displacement plethysmography (BOD POD Body Composition System; COSMED USA, Concord, CA) with the Pediatric Option and a prototype software version. In 2008–2009 COSMED USA, Inc. developed a Pediatric Option for infants/children that included a custom seat that can be secured to the testing chamber/seat of the BOD POD, a pediatric calibration cylinder (Figure 1) and Pediatric Option software. Testing occurs in the same manner as protocol dictates for adults. Briefly, subject mass is measured using an electronic scale. Subject volume is measured in an enclosed chamber utilizing the relationship between pressure and volume. When in operation, the diaphragm’s oscillations create sinusoidal volume perturbations in the two chambers that are equal in magnitude but opposite in sign. The precision of the diaphragm position is maintained by an electronic servo system. The frequency of the volume perturbation was 6 hz for subjects in this study as opposed to 3 hz for subjects > 6 years of age. Operating principles of the system have been described in detail previously (7). The volume measurement is comprised of the following steps. First, volume calibration was performed with an empty chamber and then using a known calibration cylinder (20.02 L) placed in the chamber. After calibration was completed and the testing procedures fully explained to the parent and subject, the subject entered the BOD POD system in a tight fitting swimsuit provided by the laboratory and had their total body volume measured. Unlike the body volume measurement without the Pediatric Option, the body volume measurement required three tests that lasted ≈50 seconds each with the door opened between each test. During the procedure, crying and movement were monitored (Figure 2). If crying or movement was determined to be intensive (Intensity level 3 or above) for any period during the measurement, then the measurement was deemed invalid and repeated. If two consecutive measurements were determined to be invalid then the test was cancelled.
Figure 2.
Infant crying and movement collection sheet.
Body volume was then corrected for surface area artifact and thoracic gas volume (TGV). Surface area artifact was estimated based on the following body surface area (2):
Where M is mass in kg and H is height in cm.
TGV was estimated using the following equations that were developed (COSMED USA, Inc.) using a best-fit regression to data from children aged 6–17 years ) and children aged 0–6 months (16, 17):
Where H is subject height in cm.
Total body volume and density was calculated. %fat was then calculated from whole body density with the Lohman equation (18).
Assessment of bone mineral content by dual energy X-ray absorptiometry (DXA)
A DXA measurement was performed to determine total bone mineral content using a Lunar iDXA v13.31 (GE, Fairfield, CT). All scans were analyzed using pediatric whole body extended analysis enCore 2010 software. A DVD player was placed outside of the scanning field on the scanning bed. If excessive movement occurred (indicated by an “e” in the body composition analysis portion of the iDXA software), the scan was deemed invalid, with the infant not re-scanned. The same person (DAF) positioned and analyzed all infant scans.
Assessment of total body water by deuterium dilution
Total body water (TBW) was measured using deuterium labeled water (19, 20). After a pre-dose urine sample was obtained, each child was given an oral dose of 0.2 g 99.8% D2O per kg body weight (Sigma-Aldrich; St. Louis, MO). The first urine passed after a minimum equilibration period of 3 hours was voided, the following sample as well as those obtained after 24, 48 and 72 hours were taken and frozen at −20°C until analysis. Tracer enrichment (2H:1H) were measured with isotope ratio mass spectrometry (PDZ Europa ANCA 20-20, Cheshire, U.K.) using platinum coated sticks as a catalyst (21) and the following standards: Standard Mean Ocean Water (SMOW), Standard Light Antarctic Precipitation (SLAP) and Greenland Ice Sheet Precipitation (GISP) (all purchased from the International Atomic Energy Agency, Vienna, Austria). Samples were assayed in triplicate.
To exclude data distortion due to incomplete tracer equilibration in non-compliant subjects, isotope dilution space was therefore determined using a multi-point non-linear regression incorporating the 2H:1H enrichments from all data points available. Pool sizes were assumed to be constant during the sampling period (22).
Calculation of %fat using the 4-compartment model
The 4-compartment model by Lohman (14) was used as the criterion method to which ADP was evaluated against. The Lohman 4-compartment model equation is as follows:
Where: BV is body volume by ADP (liters); TBW is total body water (liters); Mo is bone mineral content (kg); and BM is body mass (kg). Fat mass was then converted to %fat: %fat = (fat mass/BM) x 100.
STATISTICS
The accuracy, precision, bias, and reliability for ADP were examined. %fat by the Lohman 4-compartment model was selected as the criterion method because this multi-compartment model is appropriate for the population being studied (14).
Linear regression was utilized to determine the accuracy of ADP. Via linear regression we tested the hypothesis that the slope of the regression of %fat by the 4-compartment model on %fat by ADP deviated from the line of identity (i.e. that the slope was not one) and also tested the hypothesis that the intercept was different from zero.
The precision of ADP was assessed using R2 and standard error of the estimate (SEE) from the linear regression analyses with both the slope and intercept as free parameters.
Potential bias between the %fat by ADP and the %fat by the criterion method (4-compartment model) was examined by using a Bland Altman plot. Specifically, we assessed whether the difference in %fat between ADP and the 4-compartment model varied as a function of the mean %fat of ADP and the criterion method. 95% confidence intervals around the mean absolute difference between %fat by ADP and % fat by the 4-compartment model were also calculated to assess the range of agreement. A non-significant correlation suggests a lack of evidence for bias in ADP across the range of fatness (23).
The reliability of ADP was assessed using the coefficient of variation (CV), within-subject standard deviation (SD), and Cronbach’s alpha (note: Cronbach’s alpha is a particular form of an intra-class correlation) between the first and second tests (24).
Statistical analyses were performed using SPSS 18.0 (SPSS Inc., Chicago, IL) with statistical significance set at (p < 0.05; 2-tailed).
Sample Size Calculations
The number of subjects needed in Phase II (validation) was determined using the following rationale. To detect a true mean difference between %fat measured by ADP and %fat measured by the 4-compartment model using a mean difference of 2.0 %fat with a standard deviation of 3.0 %fat, at a significance level of 0.05 and 80% power, a sample size of 28 subjects whom completed all aspects of the study was required. With an expected 50% attrition/non-compliance rate, the total number of subjects required was 60 to evaluate the accuracy, precision, bias, and reliability of the new ADP pediatric option. A 50% attrition/non-compliance rate was assumed given the difficulty in obtaining valid tests results and children/parents following subject protocol in Phase I of the study.
RESULTS
A total of 42 subjects were tested in Phase II (validation) of the study. 5 subjects did not complete DXA tests and 6 subjects did not complete tests in the ADP system or did not meet the protocol’s acceptance criteria for volume measurements (in both cases due to excessive crying) and therefore excluded from analysis. The physical characteristics of the remaining 31 subjects are presented in (Table 1).
Table 1.
Physical Characteristics (n=31)
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (yr) | 4.1 ± 1.2 | 2.1 – 6.0 |
| Body Weight (kg) | 17.5 ± 3.4 | 12.0 – 25.4 |
| Height (cm) | 103.3 ± 10.2 | 84 – 125 |
| Gender (girls/boys) | 17/14 | |
| %fat from ADP | 25.6 ± 4.1 | 18.4 – 33.7 |
| 4-compartment model %fat | 26.0 ± 4.2 | 18.7 – 36.3 |
| 2 – 3 yr | n = 9 | |
| 3 – 4 yr | n = 5 | |
| 4 – 5 yr | n = 9 | |
| 5 – 6 yr | n = 8 |
Values are means ± standard deviation (SD)
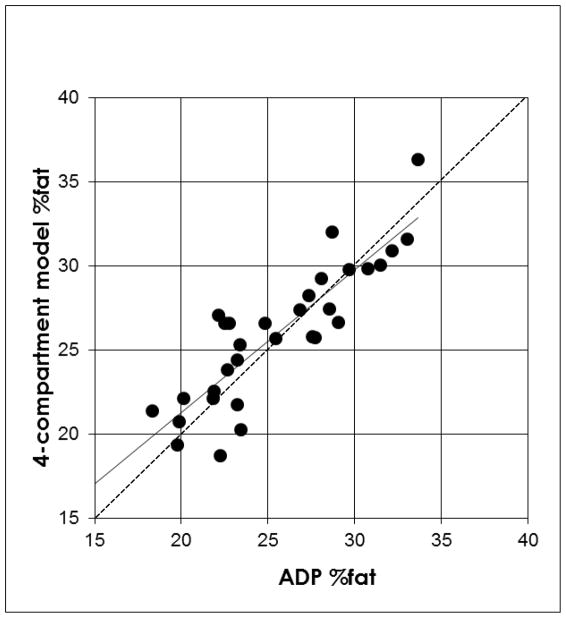
The accuracy of %fat was examined by the regression of %fat by the 4-compartment model against %fat by ADP and is shown in (Figure 3). The regression for %fat by ADP versus %fat by the 4-compartment model did not deviate from the line of identity where: y = 0.849(x) + 4.291.
Figure 3.
The regression of %fat by 4-compartment model against the %fat by ADP. The dotted line is the line of identity (regression intercept=0 and regression slope =1). The regression line (y = 0.849(x) + 4.291; R2 = 0.75 and SEE = 2.09%) did not significantly deviate from the line of identity.
The precision of ADP was determined from the R2 and the SEE from the regression analysis. ADP explained 75.2% of the variance in %fat by the 4-compartment model while the SEE was 2.09 %fat.
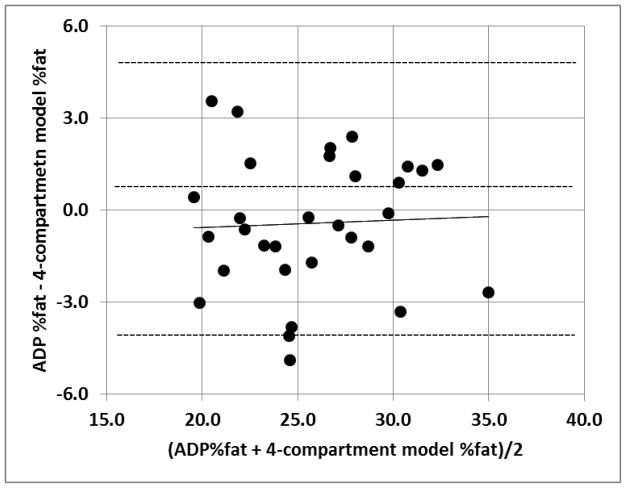
A Bland Altman was performed to assess for bias between ADP %fat and %fat by the 4-compartment model (Figure 4). The Bland Altman showed %fat by ADP did not exhibit any apparent bias across the range of fatness (r = 0.04; p = 0.81).
Figure 4.
The Bland Altman. The middle dashed line represents the mean difference between %fat from ADP − %fat from the 4-compartment model and the upper and lower dashed line represents ± 2 SD from the mean. The solid line is the regression line. No bias between the techniques was observed, as indicated by a non-significant p value (p = 0.81).
The reliability of ADP was determined by the CV, within-subject SD, and Cronbach’s alpha. The CV was 3.5%, the within-subject SD was 0.9%, and the Cronbach’s alpha was 0.95.
DISCUSSION
This study showed that the BOD POD with the Pediatric Option is valid for body composition assessment in 2 to 6 year old infants/children. Prior to the Pediatric Option, a gap existed between ≈6 months (the age at which the PEA POD can no longer accommodate subjects due to weight limitations) and ≈6 years (the age at which the BOD POD can begin testing subjects). This study showed that the new Pediatric Option interface can accurately and precisely test subjects starting at 2 years of age. The Pediatric Option now narrows the gap where ADP body composition assessment remains unproven (i.e. 6 months to 2 years of age) an improvement over the previous gap that existed.
An advantage of the Pediatric Option is that young children can be placed inside the testing chamber without worry of falling out. The Pediatric Option includes a custom seat that secures easily into the existing slats along the back of the testing chamber of any BOD POD GS model (November 2007 or newer model). The custom seat and tray is adjustable and fits snugly next to the child’s stomach, thus ensuring the subject will not fall or slide out during testing. Because the seat can be secured into place, testing a wide range of subjects is seamless allowing researchers and clinicians to effortlessly test both adult and pediatric populations in the same day. The capacity of the Pediatric Option is shown in (Table 2).
Table 2.
Capacity of Pediatric Options.
| 5th% 5-month Female |
95th% 6-year Male |
|
|---|---|---|
| Weight (kg) | 5.5 | 27 |
| Shoulder Width (cm) | 17.8 | 28.2 |
| Chest Width (cm) | 14.7 | 19.1 |
| Hip Width (cm) | 14.7 | 23.4 |
| Thigh Thickness (cm) | 5.6 | 10.7 |
TGV was estimated since subjects under 2 years of age cannot perform the “panting maneuver” which is required to measure TGV. The panting maneuver requires the subject to make two light puffs into a tube while the end is occluded (25). Moreover, the standardization in protocol was important to avoid situations where TGV was measured in some subjects and estimated in others. However, existing TGV prediction equations did not apply to the population under study. The TGV prediction equation used in children and adolescents between the age of 6 and 17 years of age provided physiologically impossible values in children less than 6 years of age. Therefore, after a review of the existing literature, we developed our own model since there was no previous data in this age range. We have used TGV data in infants < 6 months (26, 27) along with TGV data in children > 6 years (15) to develop a smoothed TGV equation for children aged 2–6 years. We realize the limitations of this approach but both TGV data < 6 months and TGV data > 6 years have proved robust in helping estimate body composition. We have no reason to believe otherwise in this age range (2–6yr) as well. Moreover, this approach provides continuous transition in measuring body composition in the PEA POD, Pediatric Option and BOD POD. Consequently, a new equation was developed by COSMED USA, Inc. using regression in infants aged 0 to 6 months and in children/adolescents aged 6 years to 17 years. This new equation incorporated in the Pediatric Option software now provides TGV estimation equation in infants, children and adolescents.
Although the original goal of the study was to validate ADP technology in infants/children between 6 months and 6 years of age, based on experience gained during the β-testing phase, we decided to limit validation of the Pediatric Option to subjects between 2 to 6 years of age. During the β-testing phase, a total of 32 subjects were enrolled, a majority of which were between 6 months to 2 years of age. Stillness in subjects between 6 months and 2 years of age, specifically intense crying, was a major issue and directly affected reliability of volume measurements. Interestingly, once a child started crying it was difficult to stop the crying without removing them from the chamber and aborting the test. If the child was placed back in the chamber, the crying would re-commence. We hypothesize that this intense crying was due to child developmental issues. Stranger and separation anxiety are powerful emotional stressors starting at 7 months and lasting until 3 years old (28). In all probability, some children in this age range will prove challenging to test in the BOD POD with the Pediatric Option.
Another potential limitation is the size of the testing chamber relative to the size of the infant/child. Gnaedinger et al reported the ratio of the testing chamber volume to subject volume should ideally be below 6 (29). This is an often overlooked, but important issue related specifically to ADP. In the β-testing phase of the study different scenarios were performed to reduce this ratio by adding volume plugs that effectively reduced the test chamber volume. Interestingly, this did not significantly affect the subject volume measurements. At this time reducing the testing chamber to subject volume ratio is neither viable nor practical and, based on our findings, might not be necessary in the population studied.
In conclusion, this study examined the reliability, accuracy, precision, and bias of the ADP pediatric option relative to the 4-compartment model in infants and small children. This is important because prior to the Pediatric Option, subjects between 6 months to 6 years of age were being lost to follow-up or were unable to have body composition assessed using ADP technology. The BOD POD with Pediatric Option was valid and reliable in infants and small children between 2 and 6 years of age, thus allowing for the continuity of testing from birth (i.e. PEA POD), to childhood (i.e. BOD POD with Pediatric Option), and on into adulthood (i.e. BOD POD) utilizing air-displacement plethysmographic principles and technology.
Acknowledgments
This work was supported by COSMED USA, Inc and by NIDDK Grant: P30DK056336. The opinions expressed are those of the authors and not necessarily those of the NIH or any other organization with which the authors are affiliated. Christoph Fusch (McMaster University) performed the D20 analyses. Michelle Gray, Brittney Criswell, and Catherine Wolf served as study coordinators.
REFERNCES
- 1.Fields DA, Goran MI. Body composition techniques and the four-compartment model in children. J Appl Physiol. 2000;89:613–620. doi: 10.1152/jappl.2000.89.2.613. [DOI] [PubMed] [Google Scholar]
- 2.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 3.Fields DA, Higgins PB, Radley D. Air-displacement plethysmography: here to stay. Curr Opin Clin Nutr Metab Care. 2005;8:624–629. doi: 10.1097/01.mco.0000171127.44525.07. [DOI] [PubMed] [Google Scholar]
- 4.Fields DA, Hunter GR. Monitoring body fat in the elderly. application of air-displacement plethysmography. Curr Opin Clin Nutr Metab Care. 2004;7:11–14. doi: 10.1097/00075197-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Ma G, Yao M, Liu Y, Lin A, Zou H, Urlando A, Wong WW, Nommsen-Rivers L, Dewey KG. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr. 2004;79:653–660. doi: 10.1093/ajcn/79.4.653. [DOI] [PubMed] [Google Scholar]
- 6.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53:486–492. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 7.McCrory MA, Gomez TD, Bernauer EM, Molé PA. Evaluation of a new air displacement plethysmograph for measuring human body composition. Med Sci Sports Exerc. 1995;27:1686–1691. [PubMed] [Google Scholar]
- 8.Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmograph for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr. 2003;77:364–370. doi: 10.1093/ajcn/77.2.364. [DOI] [PubMed] [Google Scholar]
- 9.Wells JC, Fuller NJ, Wright A, Fewtrell MS, Cole TJ. Evaluation of air-displacement plethysmography in children aged 5–7 years using a three-component model of body composition. Br J Nutr. 2003;90:699–707. doi: 10.1079/bjn2003930. [DOI] [PubMed] [Google Scholar]
- 10.Yao M, Nommsen-Rivers L, Dewey K, Urlando A. Preliminary evaluation of a new pediatric air displacement plethysmograph for body composition assessment in infants. Acta Diabetol. 2003;40 (Suppl 1):S55–58. doi: 10.1007/s00592-003-0027-9. [DOI] [PubMed] [Google Scholar]
- 11.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 12.Hull HR, Dinger MK, Knehans AW, Thompson DM, Fields DA. Impact of maternal body mass index on neonate birthweight and body composition. Am J Obstet Gynecol. 2008;198:416, e411–416. doi: 10.1016/j.ajog.2007.10.796. [DOI] [PubMed] [Google Scholar]
- 13.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol. 2006;195:1100–1103. doi: 10.1016/j.ajog.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Lohman TG. Advances in Body Composition Assessment. Champaign: Human Kinetics Publishers; 1992. [Google Scholar]
- 15.Fields DA, Hull HR, Cheline AJ, Yao M, Higgins PB. Child-specific thoracic gas volume prediction equations for air-displacement plethysmography. Obes Res. 2004;2:1797–1804. doi: 10.1038/oby.2004.223. [DOI] [PubMed] [Google Scholar]
- 16.Stock J, Sly PD, Tepper RS, Morgan WJ, editors. Measurements during tidal breathing. New York: Wiley-Liss; 1996. [Google Scholar]
- 17.Stock J, Sly PD, Tepper RS, Morgan WJ, editors. Plethysmographic assessment of functional residual capacity and airwy resistance. New York: Wiley-Liss; 1996. [Google Scholar]
- 18.Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Reviews. 1986;14:325–357. [PubMed] [Google Scholar]
- 19.Koehler K, Braun H, De Marees M, Fusch G, Fusch C, Mester J, Schaenzer W. Parallel assessment of nutrition and activity in athletes: validation against doubly labelled water, 24-h urea excretion, and indirect calorimetry. J Sports Sci. 2010;28:1435–1449. doi: 10.1080/02640414.2010.513482. [DOI] [PubMed] [Google Scholar]
- 20.Eiholzer U, Meinhardt U, Rousson V, Petro R, Schlumpf M, Fusch G, Fusch C, Gasser T, Gutzwiller F. Association between short sleeping hours and physical activity in boys playing ice hockey. J Pediatr. 2008;153:640–645. 645, e641. doi: 10.1016/j.jpeds.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Meyer H, Schonicke L, Wand U, Hubberten HW, Friedrichsen H. Isotope studies of hydrogen and oxygen in ground ice-experiences with the equilibration technique. Isotopes Environ Health Stud. 2000;36:133–149. doi: 10.1080/10256010008032939. [DOI] [PubMed] [Google Scholar]
- 22.Elia M. Technical recommendations for use in Humans NAHRES-4. Vienna: International Atomic Energy Agency (IAEA); 1990. The doubly-labelled water method for measurin energy expenditure; pp. 193–211. [Google Scholar]
- 23.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;8:307–310. [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Cronbach’s alpha. BMJ. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dempster P, Aitkens S. A new air displacement method for the determination of human body composition. Med Sci Sports Exerc. 1995;27:1692–1697. [PubMed] [Google Scholar]
- 26.Stock J, Sly PD, Tepper RS, Morgan WJ, editors. Infant respiratory function testing. New York: Wiley-Liss; 1996. [Google Scholar]
- 27.Stock J, Sly PD, Tepper RS, Morgan WJ, editors. Infant respiratory function testing. New York: Wiley-Liss; 1996. [Google Scholar]
- 28.Pediatrics AA. Caring for your baby and young child: Birth to age 5. New York: Bantam Books; 1993. [Google Scholar]
- 29.Gnaedinger RH, Reineke EP, Pearson AM, Van Huss WD, Wessel JA, Montoye HJ. Determination of body density by air displacement, helium dilution, and underwater weighing. Ann NY Acad Sci. 1963;110:96–108. doi: 10.1111/j.1749-6632.1963.tb17077.x. [DOI] [PubMed] [Google Scholar]