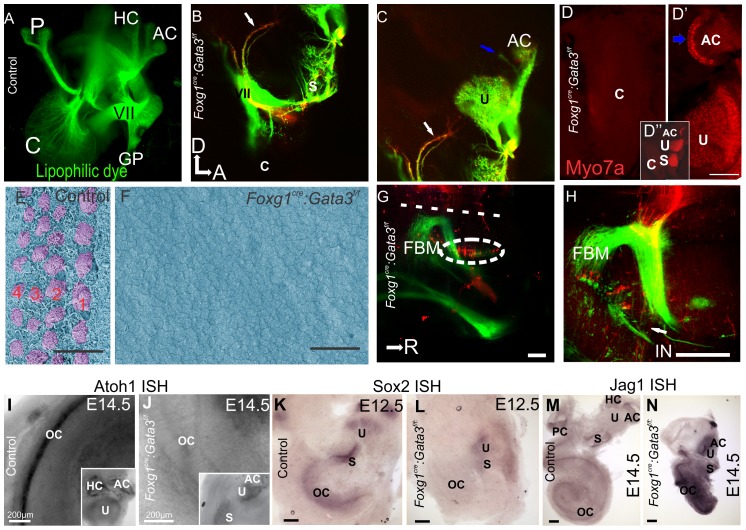
Figure 3. Foxg1Cre∶Gata3f/f mice show loss of cochlear neurosensory epithelia.
A) E16.5 Control ear showing lipophilic dye tracing of normal wild-type distribution of efferent, VII, and GP nerves. A portion of the posterior projection of the VII nerve was removed in order to visualize the cochlear (C) afferents. Injection of dye was in ipsilateral rhombomere 4. B,C) Injection of lipophilic dye in E16.5 Foxg1Cre:Gata3f/f hindbrain to label fibers to the ear as in (A), except in addition red lipophilic dye was injected to label the cochlear and vestibular afferents. The facial nerve (VII) shows a normal course around the ear. No fibers are projecting towards the cochlear duct (c). Blue arrow indicates fibers projecting to a non-existent horizontal canal. White arrow indicates fibers initially projecting to the location of where a posterior canal should reside, but in its absence these fibers diverge and project to the remaining epithelia. D) Foxg1Cre: Gata3f/f ear labeled with Myo7a immunohistochemistry shown in red. Positive cells are located in the saccule, utricle, and one canal crista. There are no Myo7a positive cells in the cochlear duct. In the center of the single canal crista there is a lack of non-sensory epithelia (blue arrow). D′′) Low magnification of entire mutant ear. E) SEM of E16.5 control organ of Corti, showing the normal stereotyped pattern of four rows of hair cells (lilac). The early tectorial membrane could be visualized during preparation. F) SEM showing a flat epithelium in the Foxg1Cre: Gata3f/f ear. No hair cells could be identified in the cochlear duct. No tectorial membrane was observed during dissection. There was also a lack of microvilli on the remaining epithelia. G,H) Inner ear efferents and FBM were both labeled with red dye on the same side simultaneously where the facial nerve wraps around the ear. The contralateral FBM were labeled with green dye. G) Efferent cell bodies can be seen (as marked by the white oval). These cells were labeled from the contralateral ear. H) Efferent axons can be seen (arrow) as they diverge from the facial nerve. All aspects of efferent cell body location are in concordance with wild type phenotype. I and J) Showing Atoh1 in situ hybridization (ISH) at E14.5. Insets show positive expression remains in the vestibular portion of the ear correlating with the presence of Myo7a positive cells, and absence within the mutant cochlear duct. K and L) Showing Sox2 ISH at e12.5. Sox2 is necessary for specification of sensory epithelia in the ear. In the control ear (K) all sensory epithelia show positive expression. In the Foxg1Cre: Gata3f/f ear (L) the vestibular portion is positive for Sox2 expression while the organ of Corti shows no expression. M and N) Showing Jag1 ISH at E14.5. Jag1 is a known marker of the prosensory domain and necessary for sensory cell development in the inner ear. Jag1 expression appears more highly upregulated with respect to topology and intensity in the mutant (N) compared to control (M). Anterior is to the right and dorsal is up in all images. Scale bars C and D indicate 10 µm; G,H, K-N indicate 100 µm, I and J indicate 200 µm. A-F, dorsal is up and anterior is to the right. G,H rostral is to the right. c, cochlea; p, posterior crista; hc, horizontal crista; ac, anterior crista; VII, facial nerve; gp, greater petrosal nerve; u, uturicle; s, saccule; FBM, facial branchial motorneurons; IN, intermediate nerve; oc, organ of Corti.