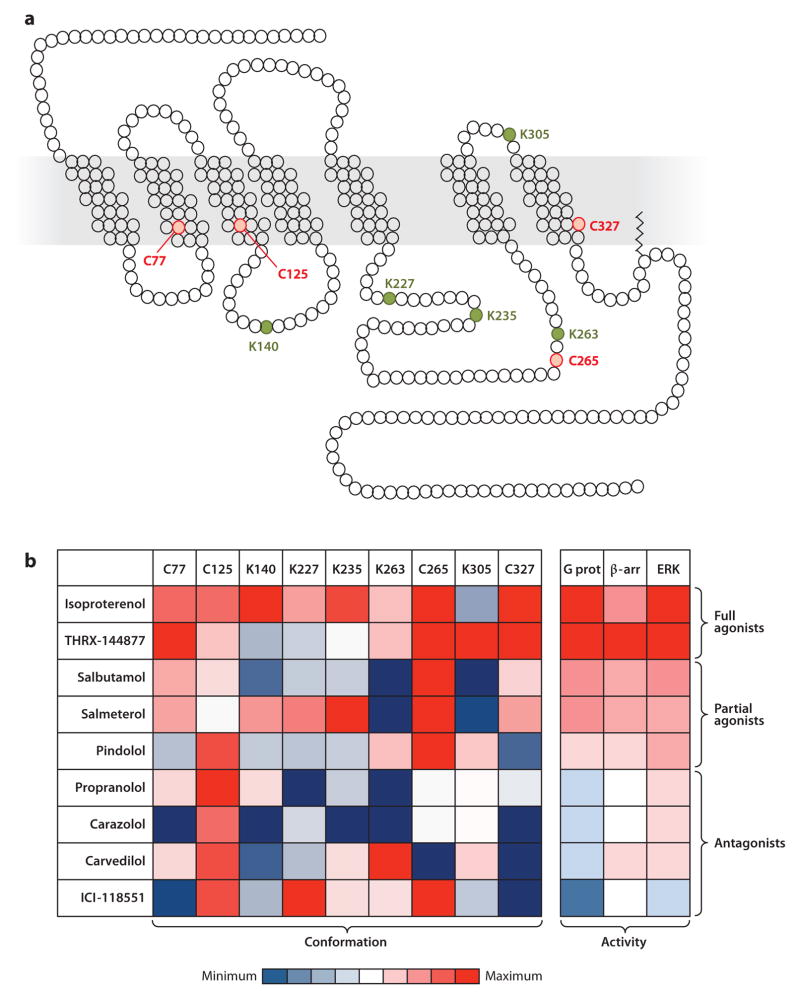
Figure 3.
Multiple ligand-specific conformations of the β2 adrenergic receptor (β2AR). Cysteines and lysines were labeled in the purified β2AR using isotope-coded N-ethylmaleimide and succinic anhydride, respectively. Mass spectrometry studies revealed that nine residues (four cysteines and five lysines) were suitable for quantifying site-specific conformational changes. (a) Schematic representation of the β2AR in single-letter amino acid code sequences showing the studied cysteines (C, red ) and lysines (K, green). (b) Labeling factors (i.e., negative log of relaxation times), measured at each of the nine cysteines or lysines upon receptor binding with nine different ligands, were plotted in a heat map (47). Values were calculated for each labeled residue upon treatment with each of the ligands; red corresponds to positive values and blue corresponds to negative values, relative to vehicle-treated β2AR. Relative activities of the nine ligands for G protein activation (G prot), β-arrestin recruitment (β-arr), and extracellular signal-regulated kinase (ERK) phosphorylation at the β2AR are represented in the heat map to facilitate direct comparison (right panel ) (47). The ligands were classified as full agonists, partial agonists, or antagonists on the basis of their pharmacological profiles.