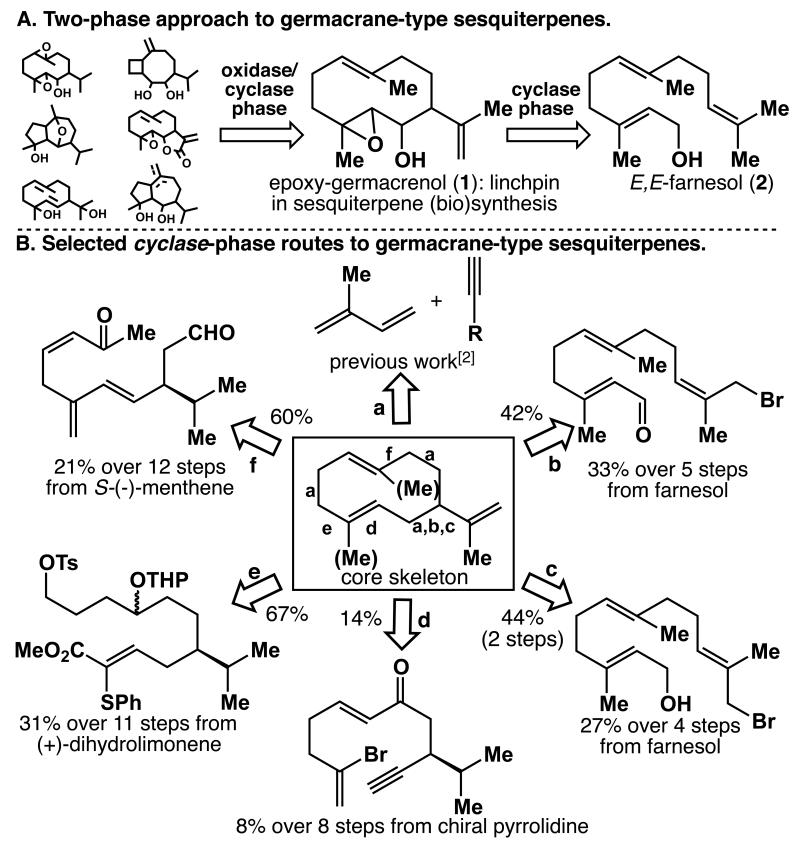
Scheme 1.
(A) Retrosynthetic outline. (B) Known cyclase-phase routes (letters indicate retrosynthetic disconnections) yielding the germacrane skeleton.[5] Cyclization conditions: a) cat. Ni(cod)2, PPh3; b) CrCl3–LiAlH4, DMF, rt, 42%; c) 1. NaH, dicyclohexano-18-crown-6, PhH, 80 °C, 2 h; 2. t-BuLi, Et2O, −78 °C, 10 h, 44% over 2 steps; d) Bu3SnH, AIBN, PhH, 80 °C, 3 h, 14%; e) NaHMDS, DME, 85 °C, 50 min, 67%; f) TiCl3, Zn/Cu, DME, 0 °C, 36 h, 60%. DME = dimethoxyethane, DMF = N,N-dimethylformamide.