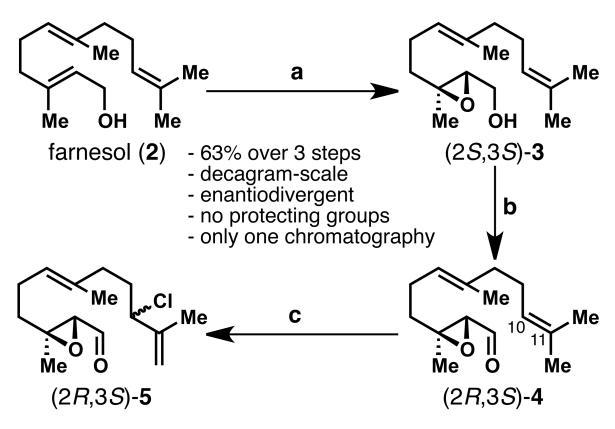
Scheme 2.
Enantioselective synthesis of the coupling precursor 5 (shown for the (2R,3S)-enantiomer). Reagents and conditions: a) 4Å MS, Ti(OiPr)4 (0.1 equiv), (+)-DET (0.12 equiv), TBHP (2 equiv), CH2Cl2, −50 °C, then 2 (1 equiv), 2 h, ee = 90%; b) SO3.py (4 equiv), iPr2NEt (5 equiv), DMSO (10 equiv), CH2Cl2, 0 °C, 0.5 h; c) PhSeCl (0.12 equiv), NCS (1.1 equiv), CH2Cl2, rt, 1 h, 63% over 3 steps. DET = diethyl tartrate, TBHP = tert-butyl hydroperoxide, DMSO = dimethyl sulfoxide, NCS = N-chlorosuccinimide.