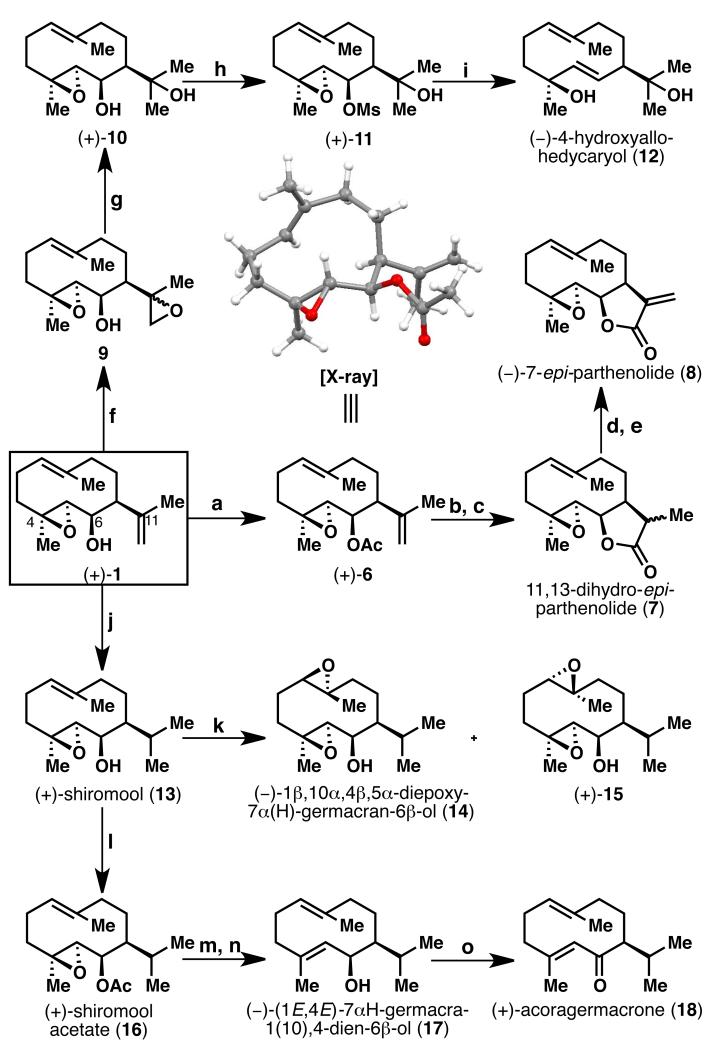
Scheme 3.
Synthesis of germacrane-type sesquiterpenes. Reagents and conditions: a) DCC (3 equiv), DMAP (0.5 equiv), AcOH (3 equiv), CH2Cl2, rt, quant.; b) 9-BBN (2 equiv), THF, rt, 2 h, then EtOH, 6N aq. NaOH, 35% aq. H2O2; c) TEMPO (0.5 equiv), PhI(OAc)2 (4 equiv), CH2Cl2, rt, 4 h, 77% over 2 steps; d) LDA (3.6 equiv), THF, −78 °C, 1 h, then CBr4 (4 equiv), 0.5 h; e) TBAF (3 equiv), THF, rt, 1 h, 60% over 2 steps; f) VO(acac)2 (0.27 equiv), TBHP (2.2 equiv), CH2Cl2, 0 °C, 77%; g) LiAlH4 (5 equiv), THF, 0 °C, 20 min; h) MeSO2Cl (3 equiv, as 2 portions), Et3N (3 equiv, as 2 portions), CH2Cl2, −5 °C, 0.5 h, 44% over 2 steps; i) Lithium naphthalenide (10 equiv, as 2 portions), THF, −25 °C, 20 min, 65 % of 12, 23% of 10; j) Crabtree’s cat. (7.5 mol %), 2,6-di-t-butylpyridine (1 equiv), CH2Cl2, H2 (1 atm), rt, 1 h, 65-84%; k) mCPBA (1.5 equiv), NaHCO3 (2 equiv), CH2Cl2, −5 °C, 30 min, 62% of 14, 22% of 15; l) DCC (3 equiv), AcOH (3 equiv), DMAP (0.5 equiv), CH2Cl2, rt, 2 h, quant.; m) WCl6 (1.85 equiv), n-BuLi (3.8 equiv), THF, −60 °C to rt, 86%; n) K2CO3 (10 equiv), MeOH, 50 °C, 1 h, 95%; o) IBX (4 equiv), DMSO, rt, 2 h, 90%. DCC = N,N’-dicyclohexylcarbodiimide, DMAP = 4-dimethylaminopyridine, BBN = borabicyclo[3.3.1]nonane, TEMPO = (2,2,6,6-tetramethylpiperidin-1-yl)oxyl, LDA = lithium diisopropylamide, TBAF = tetra-n-butylammonium fluoride, TBHP = tert-butyl hydroperoxide, mCPBA = meta-chloroperbenzoic acid, IBX = 2-iodoxybenzoic acid.