Table 1.
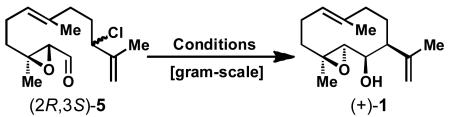
Key ring closure of 5 via an umpolung allylation.
| # | catalyst | ligand | reduct. | solv. | base | 1[a] |
|---|---|---|---|---|---|---|
| 1 | Pd(PPh3)4[b] | -- | Et2Zn[c] | THF | -- | 19 |
| 2 | Pd(PPh3)4[b] | -- | Et2Zn | THF | -- | 10-30 |
| 3 | PdCl2(PPh3)2 | -- | Et2Zn | THF | K2CO3 | 13-42 |
| 4 | PdCl2(PPh3)2 | -- | Et2Zn | DMF | K2CO3 | 32 |
| 5 | PdCl2(PPh3)2 | -- | Et2Zn | DMA | K2CO3 | 42 [d] |
| 6 | PdCl2(PPh3)2 | -- | Et2Zn | DMA | -- | 33 |
| 7 | PdCl2(PPh3)2 | -- | Me2Zn | DMA | K2CO3 | 0 |
| 8 | PdCl2(PPh3)2 | -- | Bu2Zn | DMA | K2CO3 | 31 |
| 9 | Pd(OAc)2 | PPh3 | Et2Zn | DMA | K2CO3 | 15 |
Standard conditions unless otherwise stated: cat. (10 mol %), ligand (20 mol %), K2CO3 (1.5 equiv), solvent (0.033 M overall), 5 (100–600 mg scale, 1.0 equiv., 0.05 M, slow addition over 1.5 h), reductant (1.5 equiv., slow addition over 1.5 h), T = 50 °C.
Isolated yield.
Cat. (20 mol %).
4 Equiv of Et2Zn.
Reproduced on six occasions, 42% was obtained for gram-scale.
THF = tetrahydrofuran, DMF = N,N,dimethylformamide, DMA = N,N-dimethylacetamide.
