Abstract
Background
This aim of this study was to investigate the effects of one-side cervical sympathetic block on early inflammatory response in severe trauma patients.
Material/Methods
Thirty severe trauma patients with injury severity score (ISS) of 16 to 25 were randomly divided into treatment and control groups (n=15 each). Patients in the treatment group underwent a right-side stellate ganglion block (SGB) using 8 mL 0.75% ropivacaine for 4 times, with the first injection within 12 hr of admission and the other 3 injections were 12 hr, 24 hr and 48 hr later. The same procedures were performed for the control group except that normal saline was injected instead of ropivacaine. Blood was collected before injection and at 6 hr, 24 hr, and 72 hr after the first SGB for serum interleukin (IL)-1β, IL-4, IL-6, IL-10 and TNF-α measurement.
Results
The concentrations of IL-1β, IL-6, and TNF-α between 24 hr to 72 hr after SGB were all significantly lower than those in the control group (all P values <0.01). However, there was no significant difference in the concentrations of anti-inflammatory IL-4 and IL-10 between treatment and control groups. There was no obvious impact of SGB on breathing and circulation except for a slower heart rate 10 to 50 min after injection (P<0.01).
Conclusions
SGB regulates early inflammatory response through inhibition of the proinflammatory cytokines IL-1β, IL-6, and TNF-α during severe trauma. SGB has no impact on the levels of anti-inflammatory cytokines IL-4 and IL-10.
Keywords: trauma, inflammation, stellate ganglion block, sympathetic nerve block, interleukin, TNF-α
Background
Systemic inflammatory response syndrome (SIRS) occurring during the early phase of severe trauma is one of the most important causes of complications and even death. The complicated pathophysiologic response of the neuro-endocrine-immune network involved in the mechanism of SIRS induced by trauma plays a key role in the development of and recovery from trauma[1–3]. The primary task for trauma medicine is to provide effective regulation, preventing early injury and death, and facilitating subsequent therapy and organ recovery.
The cytokine response plays a critical role in the development of SIRS in that pro-inflammatory cytokines (IL-1β, TNF-α, IL-6, IL-8, IL-12, IL-18, G-CSF and GM-CSF) are released excessively. With the significant increase of pro-inflammatory cytokines during the initial phase of trauma, anti-inflammatory cytokines, including IL-4, IL-10, IL-11 and IL-13, are usually released to down-regulate the pro-inflammatory arm of the immunity alterations. Normally, the production of pro- and anti-inflammatory cytokines is in balance to maintain biological homeostasis.
Studies into the treatment for SIRS have been focused on targeting anti-inflammatory factors through TNF-α-converting enzyme inhibition, gene therapy, blood purification, and other approaches. Numerous studies have demonstrated that anti-inflammatory therapies that target a single factor lack efficacy in clinical practice due to the complicated network composed of inflammatory factors and the cascade effects induced by the over-release of inflammatory factors. Nuclear factor-κB (NF-κB) has attracted much attention in gene therapy studies and has been regarded as the new potential target in anti-inflammatory studies. However, there is so far no report of the clinical application of NF-κB regulation. Blood purification, which includes blood filtration and blood plasma replacement, is an effective therapy for the treatment on SIRS and multiple organ dysfunction syndrome (MODS) by specifically clearing up many kinds of inflammatory factors. The principle of blood purification is to eliminate excessive cell factors and rebuild the body’s immunity to gain control. However, there are limitations of blood purification, and the technique itself needs to be improved. The duration, blood flow volume, the area and diameter of the filter should be optimized; the balance of anti-inflammation and pro-inflammation response/immunity cannot be controlled; and the production and release of inflammatory factors cannot be regulated. All of these limitations limit the curative efficacy of blood purification. In addition, blood purification is expensive and the equipment demands stringent management and maintenance, which hinders the wide application of this therapy.
As a mature and effective therapy, stellate ganglion block (SGB) has been used in the treatment of angina pectoris, bronchial asthma, gastritis, dysmenorrhea, primary hypertension, scleroderma and nearly 100 other diseases related to multiple organs, in which the neuro-endocrine-immune system is considered to be involved [4–7]. Recent animal studies indicate that SGB reduces the mortality of mice with burn injuries [8], suggesting that SGB may be useful in the regulation of early stress reaction induced by trauma. Therefore, we speculate that SGB could aid in the recovery of multi-trauma patients.
In the present study, SGB was performed on severe trauma patients, and the impact of SGB on vital signs and inflammatory factor levels of SIRS during the early phase of severe trauma were investigated.
Material and Methods
Patients
Enrollment and grouping of patients
The protocol of this study was approved by the Ethics Review Board of Southwest Hospital. Written consent was given by the patients or their next-of-kin relatives before treatment. According to the enrollment and exclusion criteria, severe multi-trauma patients who were enrolled from March to December, 2009 into the First Aid and Trauma Center were randomly divided into the SGB treatment group or the saline group (control).
The enrollment criteria
Trauma patients, men or women between 20–60 years old, meeting the following criteria were enrolled: patients with clear past medical history, no severe basic diseases such as coronary heart disease, hypertension, diabetes, chronic obstructive pulmonary disease, etc.; no history of administration of adrenergic receptor-related medications; multi-trauma with injury severity score (ISS) of 16~25; duration between time of injury to admission did not exceed 12 hr; blood pressure and breathing could be maintained without drugs or respirator; and Glasgow Coma Scale >12.
Exclusion criteria
Trauma patients with the following criteria were excluded: women who were pregnant or nursing; patients with other severe chronic diseases such as hepatic, kidney, and cardiovascular diseases; patients with psychiatric disorders or severe nervous breakdown; patients who required surgery as treatment; patients with poor compliance during treatment; patients with failed SGB; and patients with bleeding tendency.
SGB procedure
The severe trauma patients who were enrolled according to the enrollment criteria were hospitalized in the trauma intensive care unit (TICU). Electrocardiogram, blood pressure, and oxygen saturation were monitored and other conventional treatments (fluid infusion, blood transfusion, diuresis, dehydration, maintenance of fluid, electrolyte and acid-base, antibiotics) were administered as routine procedure. The patients were randomly divided into treatment or control groups. During the procedure, the patients were in supine position with a thin pillow beneath the neck to facilitate locating the tuberosity on the transverse processes of the 6th cervical vertebra, which marked the point for needle insertion. After skin antisepsis, the right tuberosity from the transverse processes of the 6th cervical vertebra was located by pressing the left thumb on the sternocleidomastoid muscle, common carotid artery, internal carotid vein, and sternohyoid muscle to the lateral side from the inner edge of the sternocleidomastoid muscle and 1 to 2 centimeters below the thyroid cartilage. With the left thumb pressing the tuberosity, a size 7 puncture needle with a connecting tube was inserted vertically from the edge of the thumb to the tuberosity. Eight milliliters of 0.75% ropivacaine (saline in control group) was injected if there was no back-flow of blood or cerebrospinal fluid after the needle reached the transverse processes. The needle was pumped back from time to time during the injection. The injection was stopped and patient was excluded if there was back-flow of blood or cerebrospinal fluid. The needle was withdrawn after the injection and the injected position was compressed for 4 to 5 min. A successful injection was marked by the appearance of Horner syndrome on the right, which included the contraction of purples, blepharoptosis, enophthalmos, blush and adiaphoresis, palpebral conjunctival hyperemia, and rhinobyon. Patients without Horner syndrome were excluded in this study. Patients were monitored by electrocardiogram and for blood pressure and oxygen saturation. Respiratory rate (RR), systolic blood pressure (SBP) and heart rate (HR) were recorded before and at the time of SGB, as well as 10 and 50 min after SGB under calm conditions. SGB was performed 4 times, with the first injection within 12 hr of admitting, and other 3 injections were 12 hr, 24 hr and 48 hr later.
Blood collection and cytokine measurements
Three milliliters of blood were collected in serum separate tubes (BD, Franklin Lakes, USA) before and at 6 hr, 24 hr, and 72 hr after the first SGB. Complete clot samples were centrifuged at 111.8 g for 1 min and kept at −70°C until analysis. Serum IL-1, IL-4, IL-6, and IL-10 were measured with BD Biosciences ELISA kits (Cat # San Jose, CA, USA) and TNF-α were tested by R&D Systems ELISA kit (Cat #, Minneapolis, MN, USA,) according to the manufacturers’ instructions. The intra-assay CV of IL-1, IL-4, IL-6, IL-10 and TNF-α is 2.6~3.3%, 3.7~6.9%, 4.1~10.8%, 2.1~2.7%, and 3.1~8.5%, respectively. The inter-assay CV is 3.11~5.86%, 2.6~7.4%, 7.9~10.9%, 5.8~10.8%, and 7.3~10.6%, respectively). The OD value of 450 nm was detected by ELISA plate reader (BIO-RAD Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All the data are expressed by χ̄±SD and were analyzed by SPSS 13.0 (Chicago, USA). The t test was used for comparison of data from corresponding time points among groups. One-way variance analysis was used for the comparison of data from different time points among groups. P values of <0.05 was considered as significant.
Results
Clinical data
According to the enrollment and exclusion criteria, 30 patients were enrolled and were randomly divided into SGB group (treatment group, n=15) and saline group (control group, n=15). All patients had severe multi-trauma and there was no significant difference in the age, sex ratio, ISS score, and time from injury to the initialization of treatment between the 2 groups (P values all >0.05) (Table 1).
Table 1.
Clinical characteristics of patients in the treatment and control groups (χ̄±SD).
| Groups | Treatment group (SGB group) (n=15) | Control group (saline group) (n=15) | P-value |
|---|---|---|---|
| Age (yr) | 38.00±10.68 | 37.40±10.98 | P>0.05 |
| Gender (male/female) | 14/1 | 14/1 | P>0.05 |
| ISS score | 19.73±3.34 | 18.62±3.34 | P>0.05 |
| Duration between injury and SGB/sham treatment (h) | 5.87±2.13 | 5.40±2.35 | P>0.05 |
Effect of SGB on respiratory function
After SGB, there was no significant difference in the respiratory rate, PH value, PaO2, PaCO2 and SpO2 of the patients (P>0.05), which indicates that SGB did not affect the respiratory function of the patients in the early phase of injury (Table 2).
Table 2.
Changes in respiratory parameters in patients before and after stellate ganglion injection (SGI) (sham or SGB) (χ̄±SD, n=15).
| Groups | Respiratory rate (/min) | pH value | P (O2) (mmHg) | P (CO2) (mmHg) | SpO2 (%) |
|---|---|---|---|---|---|
| Control group | |||||
| Before SGI | 16.5±3.3 | 7.36±0.07 | 106.8±27.0 | 35.7±3.8 | 96.7±2.1 |
| 10 min after SGI | 15.7±3.8 | 7.34±0.03 | 108.0±26.0 | 37.4±3.1 | 97.3±3.2 |
| 50 min after SGI | 16.4±3.7 | 7.35±0.04 | 107.8±25.5 | 36.7±4.9 | 96.1±2.3 |
| Treatment group | |||||
| Before SGB | 16.3±2.8 | 7.35±0.04 | 105.9±21.0 | 35.5±4.6 | 96.1±2.2 |
| 10 min after GB | 16.4±2.6 | 7.36±0.08 | 103.0±22.3 | 36.0±4.9 | 97.0±3.0 |
| 50 min after SGB | 15.9±2.8 | 7.38±0.06 | 107.9±24.0 | 36.4±4.8 | 96.5±3.5 |
Comparison within the group, or between two groups, P>0.05.
Effect of SGB on circulation parameters
There were no significant changes in systolic pressure between 10 and 50 min after SGB, or before and after SGB (P>0.05). The HR of the patients from the treatment group was significantly decreased at 10 and 50 min after SGB (P<0.01), whereas no significant difference of HR was detected before and after stellate ganglion injection (SGI) from patients in the control group (P>0.05) (Table 3).
Table 3.
Changes in circulatory function parameters before and after stellate ganglion injection (SGI) (sham or SGB) (χ̄±SD, n=15).
| Groups | SBP (mmHg) | HR (/min) | ||||
|---|---|---|---|---|---|---|
| Before SGI | 10 min after SGI | 50 min after SGI | Before SGB | 10 min after SGB | 50 min after SGB | |
| Control group | 117.5±21.3 | 115.5±24.8 | 121.8±26.5 | 116.8±13.2 | 118.9±11.7 | 116.1±8.5 |
| Treatment group | 109.5±11.2 | 105.5±10.8 | 108.2±10.7 | 115.9±10.48 | 101.6±3.6* | 104±4.6* |
P<0.01, vs. before SGB treatment.
SGB lowered serum pro-inflammatory factors
IL-1β
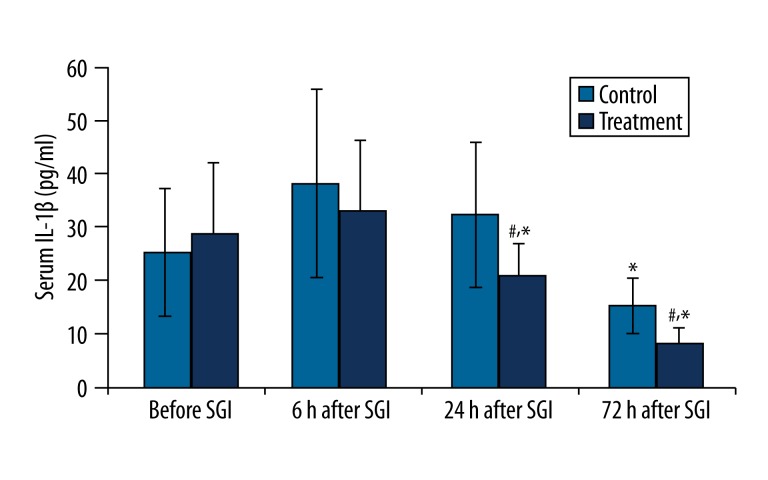
In the control group, serum IL-1β concentration demonstrated a significant decrease 72 hr after the first SGI (P<0.01). In the treatment group, IL-1β concentration was significantly decreased as early as 24 hr after SGI (P<0.01) and further decreased at 72 hr (P<0.01) after SGI. The concentrations of IL-1β at 24 hr and 72 hr after SGB in the treatment group were significantly lower than in the control group (P<0.01) (Figure 1).
Figure 1.
Changes in serum IL-1β concentration (pg/mL) before and after the first stellate ganglion injection (SGI) (sham or SGB). * P<0.01, compared to the previous time point value of the same group; #P<0.01, compared to the corresponding time point value of the control group.
IL-6
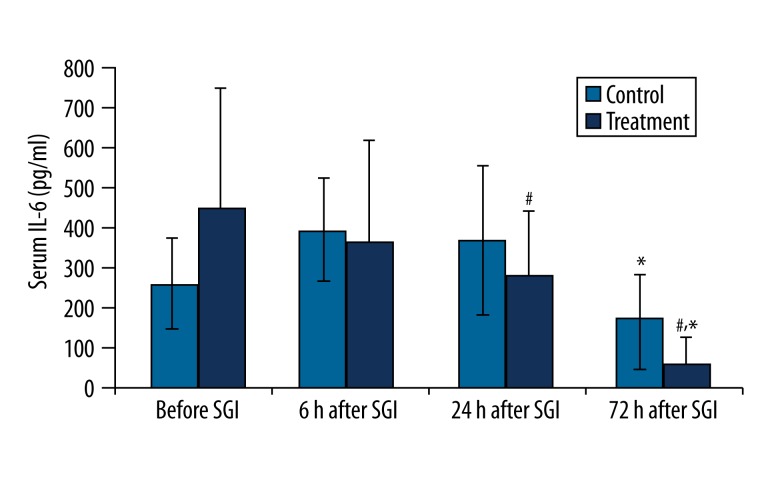
There was significant decrease in serum IL-6 concentrations at 72 hr after SGB/SGI in the treatment and control groups (P<0.01). In addition, IL-6 concentrations were significantly decreased at 24 hr and 72 hr after SGB in the treatment group compared to the control group (P<0.01) (Figure 2).
Figure 2.
Changes in serum IL-6 concentration (pg/ mL) before and after the first stellate ganglion injection (SGI) (sham or SGB). * P<0.01, compared to the previous time point value of the same group; #P<0.01, compared to the corresponding time point value of the control group.
TNF-α
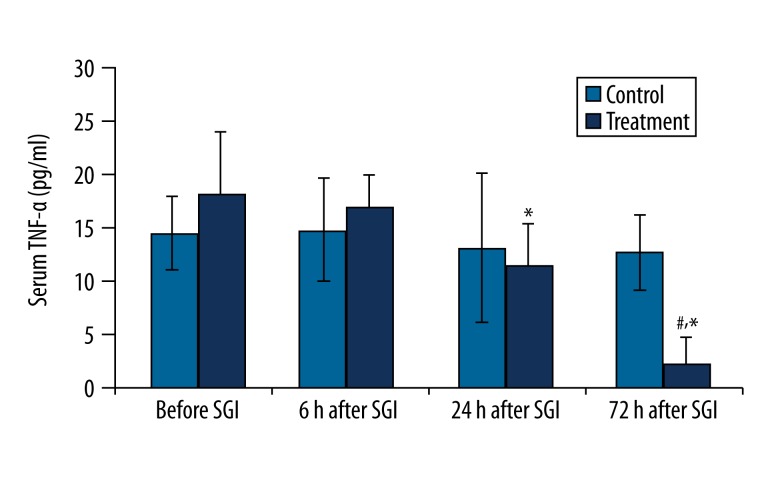
There was no change in TNF-α concentration at any time point before or after SGI in the control group. However, in the treatment group, the concentration of TNF-α was significant decreased at 24 hr and 72 hr after SGB compared to controls (P<0.01) (Figure 3).
Figure 3.
Changes in serum TNF-α concentration (pg/ml) before and after the first stellate ganglion injection (SGI) (sham or SGB). * P<0.01, compared to the previous time point value of the same group; #P<0.01, compared to the corresponding time point value of the control group.
Effect of SGB on serum anti-inflammatory factors
IL-4
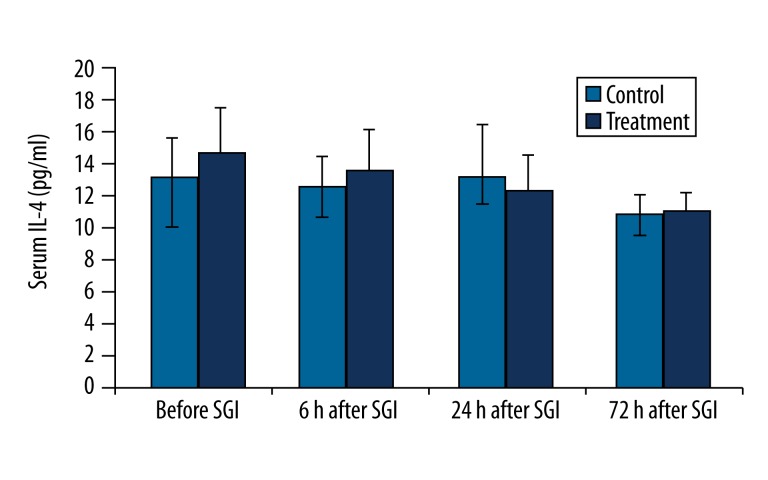
The concentration of IL-4 was not altered within 72 hr after SGI in the control group. Although the IL-4 concentration decreased slowly within 72 hr after SGB in the treatment group, the result was not statistically significant as analyzed by variance analysis. There was no significant difference in IL-4 concentration at any time point after SGB between control and treatment groups (Figure 4).
Figure 4.
Changes in serum IL-4 concentration (pg/ml) before and after the first stellate ganglion injection (SGI) (sham or SGB).
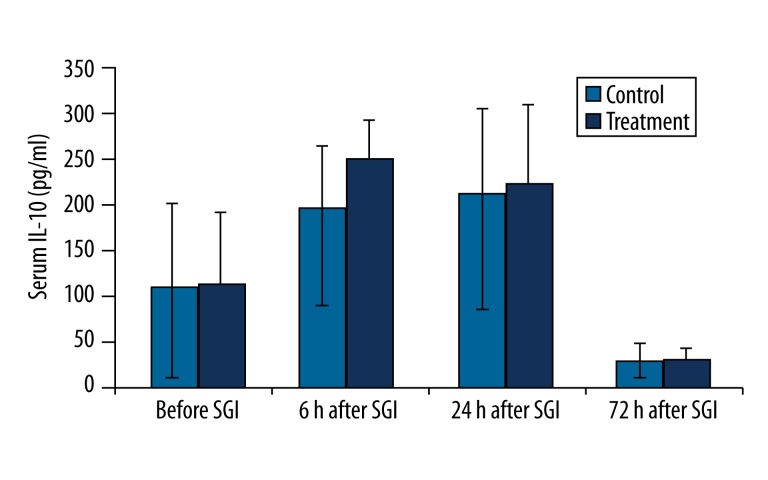
IL-10
The concentration of IL-10 was significantly decreased at 72 hr compared to 24 hr after SGB/SGI in the treatment and control groups (P<0.01). However, the concentration of IL-10 at any time point after SGB did not significantly differ in the treatment group compared with the control group (Figure 5).
Figure 5.
Changes in serum IL-10 concentration (pg/ml) before and after the first stellate ganglion injection (SGI) (sham or SGB). * P<0.01, compared to the previous time point value of the same group.
Discussion
Severe trauma is usually induced by the overreaction of stress response, involving a complicated network of the neuro-endocrine-immune system. In general, the integration and feedback of neural, endocrine and immune systems allow the body to respond to trauma in a manner aimed at resuming the homeostasis of the body system. However, this ability to protect and regulate has its own limitations. Once the body fails to react appropriately to severe trauma, the overreaction or underreaction leads to a stress reaction disorder, followed by even more severe systemic injury [9] such as SIRS. Unregulated stress/inflammatory response is considered the key initiation step in the deterioration of the disease and remains the focus of therapy [10]. If SIRS can be impeded and controlled, the success rate for the treatment of severe trauma can be greatly improved and mortality can be considerably reduced [11].
The sympathetic nervous system, which derives from the nucleus ceruleus of the brainstem, plays an important role in the interaction of neural and immune systems. The post-ganglionic sympathetic nerves, which travel through the paravertebral ganglia and prevertebral ganglia, release noradrenaline in different tissues. The concentrations of brain noradrenaline and serum cortisol are proportionately increased in order to maintain the reflex circuit of the hypothalamus-pituitary-adrenal (HPA) and sympathetic nerve systems. Under physiological conditions, the immune organs are innervated by the sympathetic nerves, and adrenergic receptors are expressed on the surface of almost all the granulocyte. Sympathetic nerve activity strongly affects immune function [12–16]. To block sympathetic nerves, the use of cervical sympathetic block has had a long history of usage and can affect the regulation of the neuroendocrine-immune network to facilitate the recovery of body homeostasis from the unbalanced stasis induced by stress.
Interestingly, the stimulation of parasympathetic nerves may also suppress systemic inflammatory response [17,18]. Our previous study demonstrated that the mortality and inflammatory response of animals may be decreased by blocking sympathetic nerves through SGB [8]. Due to their unique anatomical structure and physiological functions, the sympathetic nervous system (SNS) and HPA, which play central roles in neuroendocrine regulation, were found to have similar regulatory function [19]. The main functions of sympathetic nerves include increased contractility and rate of heartbeat, constriction of splanchnic vessels, dilation of bronchial smooth muscle, inhibition of gastrointestinal activity, and sphincter constriction. SGB restrains the activity of the central and peripheral sympathetic nerves, and corrects the pathological hyper-function of the sympathetic activity to restore normal levels and maintain homeostasis. It has been demonstrated that SGB reduces the fluctuation range of heart rate and blood pressure of rabbits during cerebral ischemia and reperfusion [20], indicating that SGB attenuates the over-excitation of the sympathetic nerve center (located in the hypothalamus) induced by stress stimulation. SGB also decreases serum IL-6 and IL-8 levels of rabbits during cerebral ischemia and reperfusion injury [21]. These animal experiments indicate a tight connection between sympathetic nerves and inflammatory factors, which exerts important impact on stress reaction.
TNF-α, IL-1β, and IL-6 are important proinflammatory factors and are important for the induction and development of SIRS. IL-1 is produced by macrophages and is one of the key cytokines in acute inflammatory response. IL-1 autoregulates its expression, as well as other proinflammatory factors, adhesion molecules, and chemotactic factors such as IL-6, IL-8 and TNF-α [22]. IL-6 induces the synthesis and release of other inflammatory factors and enhances the functions of IL-1. TNF-α is produced by monocytes and macrophages and stimulates the synthesis and release of IL-1 and IL-6 from hepatocytes, which induces the cascade effect [23]. TNF-α participates in the development of trauma, post-traumatic infection, and neurologic damage, and also predicts the prognosis of trauma [24–26]. Previous animal studies suggest that sympathetic nerve block reduces the concentration of TNF-α, IL-1β, and IL-6 in SIRS [8,27].
In the current study, we found that SGB significantly suppressed the induction of IL-1β, IL-6, and TNF-α levels caused by trauma. The concentrations of IL-1β at 24 hr and 72 hr after SGB were significantly lower in the treatment group compared to the control group (P<0.01), suggesting that early treatment of SGB significantly inhibited the synthesis/secretion of serum IL-1β. IL-6 is the index for the degree of damage in the early phase of trauma. The significant effect of SGB on the reduction of serum IL-6 concentration also appeared at 24 hr and 72 hr after SGB in the treatment group compared with the control group (P<0.01), suggesting that SGB inhibited the synthesis/secretion of IL-6. The change of serum TNF-α concentration in the control group was not obvious, whereas in the treatment group, TNF-α was significantly lower at 24 hr and 72 hr after SGB than that of the control group (P<0.01), indicating that SGB suppressed the synthesis/secretion of TNF-α, facilitated the recovery of TNF-α stasis, and might be important for the improvement of trauma prognosis [28].
The human body is a complex system with a strong sense of self-regulation. In SIRS, in order to suppress the synthesis and release of proinflammatory factors and maintain homeostasis, the body not only initiates the anti-inflammatory response (which depends mainly on the glucocorticoid receptor pathway exerting limited protection [29,30]), but also passively generates many anti-inflammatory factors such as IL-4 and IL-10. The proinflammatory factors were drastically altered after SGB, which leads to various bodily adjustments. It has been suggested that IL-4 enhances the functions of macrophages and IL-10 restrains the cellular immunologic response, facilitating the generation of antibodies and inhibiting the release of proinflammatory factors, including TNF-α and IL-6 [31]. However, in the present study we found that SGB did not significantly affect serum IL-4 and IL-10 concentrations compared with controls (P>0.05). It seems that production of proinflammatory factors but not of anti-inflammatory factors is affected by SGB. We speculate that this may be related to our intervention in the early stage, when anti-inflammatory factors generate passively and at lower levels. Further investigations in this area should be performed.
With the recent introduction of the concept of inflammasome into the inflammatory response induced by trauma, SGB regulation can be further investigated. Inflammasome is a multi-protein complex located in the cytosol [32] and is the essential platform for the activation of caspase-1. Inflammasome modulates the process and activation of many inflammatory factors such as IL-1β, IL-18, and IL-33 [33]. Nucleotide-binding and oligomerization domain-like receptors are important pattern-recognized receptors located in cytosol, which accomplish the composition of inflammatory factors of inflammasome [34] on the stimulation of stress. The body can be considered as an aggregation of many kinds of inflammasome. The aggregation is kept in balance if the stress is minor. However, if the stress becomes overwhelming, the body will initiate systemic inflammasome to fight against the immunity in order to restore homeostasis. Once the balanced status of core composition anti-inflammatory and proinflammatory factors is broken, the body can be injured by autoimmunity. Thus, no single treatment can act to restore the balanced status and may have the opposite result. SGB works through the regulation of the neuroendocrine-immune system in assisting the body to regulate and control the recovery of the balanced point of inflammasome, and hence proves to be an effective method. The sympathetic nervous system and HPA play central roles in neuroendocrine regulation. Being a key neurotransmitter of sympathetic nerves, norepinephrine may be one of the main molecules involved during the cytokine response by SGB, and its role warrants further investigations.
As expected, the breathing, blood pressure and heart rate of severe trauma patients were affected by the sympathetic nerve block. This is the primary problem to consider before initiating sympathetic nerve block. Breathing and circulation are affected in various degrees of trauma patients. The amount of blood loss, the mechanism and location of the injury, and shock condition all influence the efficacy of treatment. Therefore, we first excluded patients who required medication to maintain blood pressure or a respirator for breathing. Second, we preserved the conventional treatment of trauma. Third, the sympathetic nerve block was performed in the trauma intensive care unit in order to assure close monitoring of patients, who could be treated immediately in case of complications.
The current study demonstrates that sympathetic nerve block restored the heart rate increase induced by trauma back to normal condition, and slightly reduced systolic pressure, which was not significantly lower than before the SGB. These results are consistent with previous reports [8,19], and can be explained by the fact that sympathetic nerve block maintains the functions of vegetative nerves, normalizes the abnormal tension of vessels, and preserves normal blood pressure. In addition, our study also found that sympathetic nerve block did not affect the breathing rate or blood gases, which is consistent with the study of Parris et al [35]. Some studies have reported that the decrease in PaO2 was due to the block of phrenic nerves by the diffusion of local anesthetic drug [36], but not due to the weakness of the activity of diaphragm muscle by block of sympathetic nerves.
Conclusions
Increasing attention in clinical practice is being paid to the occurrence and development of SIRS induced by severe trauma. Studies on the mechanism of SIRS indicate that the injury to the body originated from the cascade amplification of the neuroendocrine-immune network activated by stress. Molecular studies have suggested the involvement of ILs and TNF-α in SIRS. We observed in the current study that SGB suppressed the expression of proinflammatory factors during early systemic inflammation induced by severe trauma, but the mechanism is still unclear and needs to be further investigated. Nonetheless, SGB is an effective and simple treatment that can be used as a potential therapy for SIRS.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: This study was supported by the Open Foundation from State Key Laboratory of Trauma, Burns, and Combined Injuries
References
- 1.Morris JA, Jr, Norris PR, Moore JH, et al. Genetic Variation in the Autonomic Nervous System Affects Mortality: A Study of 1,095 Trauma Patients. J Am Coll Surg. 2009;208(5):663–70. doi: 10.1016/j.jamcollsurg.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Yang FL, Subeq YM, Lee CJ, et al. Rosiglitazone protects against severe hemorrhagic shock-induced organ damage in rats. Med Sci Monit. 2011;17(10):BR282–89. doi: 10.12659/MSM.881975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sikora JP, Kuzanski W, Andrzejewska E. Soluble cytokine receptors sTNFR I and sTNFR II, receptor antagonist IL-1ra, and anti-inflammatory cytokines IL-10 and IL-13 in the pathogenesis of systemic inflammatory response syndrome in the course of burns in children. Med Sci Monit. 2009;15(1):CR26–31. [PubMed] [Google Scholar]
- 4.Crockett A, Panickar A. Role of the sympathetic nervous system in pain. Anaesthesia & Intensive Care Medicine. 2010;12(2):50–54. [Google Scholar]
- 5.Lee C-C, Chuang C-C, Liou J-Y, et al. Successful management of contrast medium extravasation injury through stellate ganglion block and intra-arterial nitroglycerin. Acta Anaesthesiol Taiwan. 2011;49:116–18. doi: 10.1016/j.aat.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.He J-Y, Jiang L-S, Dai L-Y. The roles of the sympathetic nervous system in osteoporotic diseases: A review of experimental and clinical studies. Ageing Research Reviews. 2011;10:253–63. doi: 10.1016/j.arr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Nickel FT, Seifert F, Lanz S, et al. Mechanisms of neuropathic pain. Eur Neuropsychopharmacol. 2012;22:81–91. doi: 10.1016/j.euroneuro.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Lu J, Shi Z, Su Y, et al. Effect of cervical sympathetic ganglia block on the mortality of mice with combined radiation and burn injury and its possible mechanism. Chin J Clin Rehabil. 2006;10(34):177–81. [Google Scholar]
- 9.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36(6):691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 10.Osuchowski MF, Welch K, Siddiqui J, Remick DG. Circulating cytokine/inhibitor profiles reshape the understanding of the SIRS/CARS continuum in sepsis and predict mortality. J Immunol. 2006;177(3):1967–74. doi: 10.4049/jimmunol.177.3.1967. [DOI] [PubMed] [Google Scholar]
- 11.Lausevic Z, Lausevic M, Trbojevic-Stankovic J, et al. Predicting multiple organ failure in patients with severe trauma. Can J Surg. 2008;51(2):97–102. [PMC free article] [PubMed] [Google Scholar]
- 12.Kin NW, Sanders VM. It takes nerve to tell T and B cells what to do. J Leukoc Biol. 2006;79(6):1093–104. doi: 10.1189/jlb.1105625. [DOI] [PubMed] [Google Scholar]
- 13.Mousa SA, Shaqura M, Brendl U, et al. Involvement of the peripheral sensory and sympathetic nervous system in the vascular endothelial expression of ICAM-1 and the recruitment of opioid-containing immune cells to inhibit inflammatory pain. Brain Behav Immun. 2010;24:1310–23. doi: 10.1016/j.bbi.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Ley S, Weigert A, Brune B. Neuromediators in inflammation – a macrophage/nerve connection. Immunobiology. 2010;215:674–84. doi: 10.1016/j.imbio.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Xu X, Wang P, Zou X, et al. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV1 in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol. 2010;222:93–107. doi: 10.1016/j.expneurol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellinger DL, Millar BA, Perez S, et al. Sympathetic modulation of immunity: Relevance to disease. Cell Immunol. 2008;252(1–2):27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pavlov VA, Ochani M, Gallowitsch-Puerta M, et al. Central muscarinic cholinergic regulation of the systemic inflammatory response during endotoxemia. Proc Natl Acad Sci USA. 2006;103(13):5219–23. doi: 10.1073/pnas.0600506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adachi M, Otsuki M, Akatsu M, Tase C. The effects of heat stimulation and cold stress on the rats with cervical sympathectomy. Masui. 2003;52(12):1293–99. [PubMed] [Google Scholar]
- 20.Quan SB, Liu JY, Wang QX, Yang G. Relationship between serum interleukin-6 level and stellate ganglion block in rabbit brain during ischemic-reperfusion period. Chin J Clin Rehabil. 2005;9(37):146. [Google Scholar]
- 21.Wang QX, Wang XY, Fu NA, et al. Stellate ganglion block inhibits formalin-induced nociceptive responses: mechanism of action. Eur J Anaesthesiol. 2005;22(12):913–18. doi: 10.1017/S0265021505001559. [DOI] [PubMed] [Google Scholar]
- 22.Chawda M, Hildebrand F, Pape H, Giannoudis P. Predicting outcome after multiple trauma: which scoring system? Injury. 2004;35(4):347–58. doi: 10.1016/S0020-1383(03)00140-2. [DOI] [PubMed] [Google Scholar]
- 23.Gebhard F, Pfetsch H, Steinbach G, et al. Is interleukin 6 an early marker of injury severity following major trauma in humans? Arch Surg. 2000;135(3):291–95. doi: 10.1001/archsurg.135.3.291. [DOI] [PubMed] [Google Scholar]
- 24.Krinsley JS. Association between hyperglycemia and increased hospital mortality in a heterogeneous population of critically ill patients. Mayo Clin Proc. 2003;78(12):1471–78. doi: 10.4065/78.12.1471. [DOI] [PubMed] [Google Scholar]
- 25.Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–59. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 26.Downen M, Amaral TD, Hua LL, et al. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28(2):114–27. [PubMed] [Google Scholar]
- 27.Ni Choileain N, Redmond HP. The immunological consequences of injury. Surgeon. 2006;4(1):23–31. doi: 10.1016/s1479-666x(06)80018-1. [DOI] [PubMed] [Google Scholar]
- 28.Windahl SH, Treuter E, Ford J, et al. The nuclear-receptor interacting protein (RIP) 140 binds to the human glucocorticoid receptor and modulates hormone-dependent transactivation. J Steroid Biochem Mol Biol. 1999;71(3–4):93–102. doi: 10.1016/s0960-0760(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 29.Spielmann S, Kerner T, Ahlers O, et al. Early detection of increased tumour necrosis factor alpha (TNFalpha) and soluble TNF receptor protein plasma levels after trauma reveals associations with the clinical course. Acta Anaesthesiol Scand. 2001;45(3):364–70. doi: 10.1034/j.1399-6576.2001.045003364.x. [DOI] [PubMed] [Google Scholar]
- 30.Savory JGA, Hsu B, Laquian IR, et al. Discrimination between NL1-and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19(2):1025. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007;7(1):31–40. doi: 10.1038/nri1997. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe H, Gehrke S, Contassot E, et al. Danger signaling through the inflammasome acts as a master switch between tolerance and sensitization. J Immunol. 2008;180(9):5826–32. doi: 10.4049/jimmunol.180.9.5826. [DOI] [PubMed] [Google Scholar]
- 33.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4(2):95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 34.Feldmann J, Prieur AM, Quartier P, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. 2002;71(1):198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parris WC, Lin S, Frist W., Jr Use of stellate ganglion blocks for chronic chest pain associated with primary pulmonary hypertension. Anesth Analg. 1988;67(10):993–95. [PubMed] [Google Scholar]
- 36.Hardy PA. Stellate ganglion block with bupivacaine. Minimum effective concentration of bupivacaine and the effect of added potassium. Anaesthesia. 1989;44(5):398–99. doi: 10.1111/j.1365-2044.1989.tb11337.x. [DOI] [PubMed] [Google Scholar]