Abstract
Background
Polymorphisms in genes involved in the metabolism of folate and methyl groups have been implicated with risk of digestive system cancer. Methionine synthase (MTR) plays a central role in folate metabolism, thereby affecting DNA methylation. The association between A2756G polymorphism (rs1805087) in MTR and digestive system cancer susceptibility was inconsistent in previous studies. To investigate this inconsistency, we performed this meta-analysis.
Methods
Databases including Pubmed, EMBASE, ISI Web of Science and China National Knowledge Infrastructure (CNKI) were searched to find relevant studies. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of association. Potential sources of heterogeneity were also assessed by subgroup analysis and meta-regression.
Results
A total of 29 articles with 15,368 patients and 23,959 controls were included. We found no association between MTR A2756G polymorphism and digestive system cancer in overall population (G allele: OR = 1.03, 95% CI = 0.98–1.09, P = 0.25; dominant model: OR = 1.03, 95% CI = 0.97–1.10, P = 0.33; recessive model: OR = 1.02, 95% CI = 0.89–1.17, P = 0.79). In the stratified analyses according to cancer type, sample size and genotyping method, no evidence of any gene-disease association was obtained in almost all genetic models. However, marginal significant associations were found for East Asians and hospital-based studies.
Conclusions
This meta-analysis suggests that there is no significant association between the MTR A2756G polymorphism and digestive system cancer risk.
Introduction
It is predicted that by 2020, the number of new cases of cancer in the world will increase to more than 15 million, with deaths increasing to 12 million [1]. Digestive system cancers are the most common malignant tumors worldwide, with three million new cases each year (nearly 30% of all cancers) [1], [2]. The incidence of digestive system cancers will be constantly increasing, mainly due to trends in gastric cancer (GC) and colorectal cancer (CRC) [2]. In European countries, there were an estimated 0.91 million new cases of digestive system cancers (436,000 CRC and 149,000 GC) and 0.59 million deaths from these health care problems in 2008 [3]. In the majority of developing countries, the upward trends of mortality rates for digestive system cancers also have been observed [4], [5].
Methylation of the promoter-associated CpG islands is a well-documented epigenetic modification, acting as a mechanism to regulate gene expression associated with the development of cancer [6], [7]. Aberrant methylation of the tumor suppressor or DNA repair gene promoter has been detected in many different types of cancers [8], [9]. Methionine synthase, a vitamin B 12 -dependent enzyme, plays an important role in folate metabolism [10]. It catalyzes the remethylation of homocysteine to methionine and the concurrent demethylation of 5-methyltetrahydrofolate to tetrahydrofolate. Methionine synthase is critical for maintaining adequate intracellular methionine, an essential amino acid and the precursor of S-adenosylmethionine (SAM). SAM is a crucial methyl group donor involved in over 100 methylation reactions including DNA methylation. Recently, a polymorphism in the methionine synthase (MTR) gene (2756A→G, rs1805087), resulting in the substitution of aspartic acid (D919) by glycine (G), was identified in patients with methionine synthase deficiency and was found to be polymorphic among healthy controls [11]. In addition, Goode et al. suggested a modest inverse association between 2756GG polymorphism and homocysteine levels, indicating an increased enzymatic activity of the variant genotype [12]. Furthermore, a reduced homocysteine level was linked to the GG genotype in some studies [13]–[15], leading to the hypothesis that this polymorphism may have an activating effect on the enzyme that increases the conversion of homocysteine to methionine. Moreover, Paz et al. reported that individuals who carried 2756GG showed a lower frequency of CpG island hypermethylation in tumor suppressor genes [16].
Despite the biological plausibility of MTR functional polymorphism as a modulator of digestive system cancer susceptibility, previously inconsistent results have appeared in the literature. Published studies have generally been restricted in terms of sample size and ethnic diversity, and individual studies may have insufficient power to achieve a comprehensive and reliable conclusion. We therefore performed a meta-analysis of the published studies to clarify this inconsistency and to establish a comprehensive picture of the relationship between MTR and digestive system cancer.
Materials and Methods
Identification and Eligibility of Relevant Studies
Genetic association studies published before the end of Sep. 2012 on digestive system cancer and polymorphism within MTR gene were identified through a search of PubMed, EMBASE, ISI Web of Science, and CNKI (Chinese National Knowledge Infrastructure) without language restrictions using the following keywords and subject terms: ‘methionine synthase’ or ‘MTR’, ‘polymorphism’ or ‘variation’, and ‘cancer’ or ‘carcinoma’ or ‘neoplasm’. The titles and abstracts of potential articles were screened to determine their relevance, and any clearly irrelevant studies were excluded. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. Furthermore, reference lists of primary studies and review articles were also reviewed by a manual search to identify additional relevant publications. Studies included in the meta-analysis had to meet all the following criteria: (1) original papers containing independent data, (2) case–control or cohort studies, (3) association between MTR polymorphism and digestive system cancer risk was explored (4) identification of digestive system cancer cases was confirmed histologically or pathologically and (5) genotype distribution information or odds ratio (OR) with its 95% confidence interval (CI) and P-value. The major reasons for exclusion of studies were (1) overlapping data and (2) case-only studies, and review articles.
Data Extraction
For each study, the following data were extracted independently by two authors: first author, year of publication, diagnosis criterion, age, sex, ethnicity, Hardy–Weinberg equilibrium (HWE) status, genotyping method, cancer type, source of control, total number of cases and controls, MTR polymorphism genotype counts and interactions between environmental factors or genes. The results were compared, and disagreements were discussed among all authors and resolved with consensus. If multiple published reports from the same study population were available, we included only the one with largest sample size and the most detailed information. Studies with different ethnic groups were considered as individual studies for our analyses.
Statistical Analysis
Deviation from Hardy–Weinberg equilibrium was examined by Chi-square test with 1 degree of freedom. Crude Odds ratio (ORs) with corresponding 95% confidence intervals (CIs) were used to assess the strength of association between the MTR gene A2756G polymorphism and digestive system cancer risk. For the A2756G polymorphism, we investigated the association between genetic variants and digestive cancer risk in multiplicative model (G-allele vs. A-allele), dominant (AA+AG vs. GG) and recessive genetic model (GG vs. AA+AG). Between-study heterogeneity was measured using standard Q-statistic test [17]. Random-effects and fixed-effect summary measures were calculated as inverse-variance weighted average of the log odds ratio [18]. The results of random-effects summary were reported in the text because it takes into account the variation between studies. The Z test was used to determine the significance of the pooled OR. Subgroup analysis was stratified by the study characteristic according to ethnicity (East Asian, Caucasian and other), study design (hospital-based vs population-based) sample size (≥500 or <500 cases), genotyping method (RFLP vs others) and cancer type (colorectal cancer, esophagus cancer, gastric cancer, pancreatic cancer and hepatocellular carcinoma), respectively. Furthermore, meta-regression analysis was performed to investigate seven potential sources of heterogeneity including ethnicity, sample size, source of controls, genotyping method, cancer type, sex distribution among cases and controls, mean age of cases and controls [19]. Publication bias was investigated by funnel plot. Funnel plot asymmetry was assessed by the method of Egger’s linear regression test [20]. Sensitivity analysis, which determines the influence of individual studies on the pooled estimate, was determined by sequentially removing each study and recalculating the pooled relative risk and 95% confidence interval. Statistical analyses were done with the Stata software version 10.0 (Stata Corporation, College Station, TX). The type I error rate was set at 0.05. All P-values were two-tailed.
Results
Characteristics of Studies
The combined search yielded 217 references. Study selection process was shown in Figure 1. Finally, a total of 29 studies with 34 data sets were finally included involving 15,368 patients and 23,959 controls [15], [21]–[48]. The detailed characteristics of the studies included in this meta-analysis are shown in Table 1. Of the cases, 82% were Caucasian, 16% were East Asian and 2% were other ethnic origins. The distribution of genotypes in the controls was consistent with Hardy–Weinberg equilibrium in all studies for MTR A2756G polymorphism.
Figure 1. Flow diagram of the study selection process.
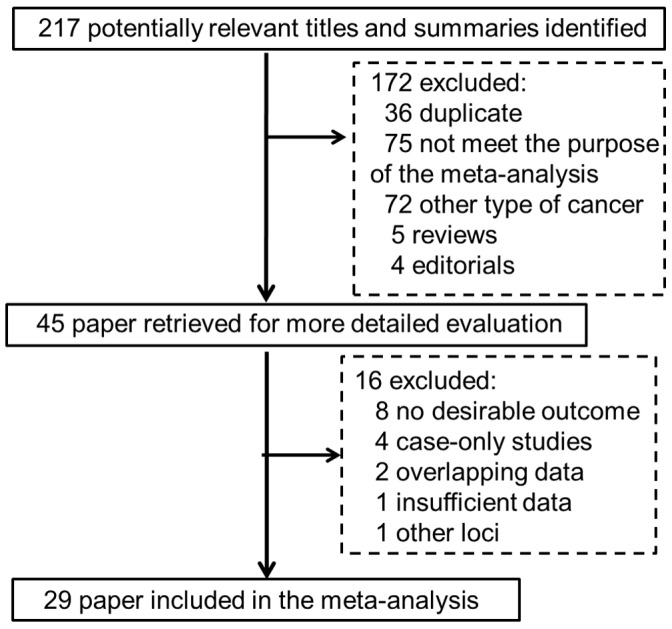
Table 1. Characteristics of the studies included in the meta-analysis.
| Study | Year | Ethnicity | Cancertype | Controlsource | No. ofcases/controls | Mean age ofcases/controls | Gender distributionin cases/controls(male %) | Genotypingmethod | PHWE forcontrols |
| Ma [15] | 1999 | American | CRC | PB | 356/476 | NA/NA | 100/100 | RFLP | 0.15 |
| Le Marchand [21] | 2002 | American | CRC | PB | 539/652 | 66.0/67.0 | 60.8/57.9 | RFLP | 0.54 |
| Matsuo [22] | 2002 | Japanese | CRC | HB | 142/241 | NA/NA | 58.9/49.0 | RFLP | 0.28 |
| Pufulete [23] | 2003 | British | CRC | HB | 28/76 | 68.9/58.0 | 46.0/45.0 | RFLP | 0.07 |
| Ulvik [24] | 2004 | Norwegian | CRC | PB | 2168/2192 | NA/NA | 63.5/63.5 | Taqman | 0.34 |
| Matsuo [25] | 2005 | Japanese | CRC | HB | 257/771 | 58.8/59.0 | 63.0/63.0 | RFLP | 0.4 |
| Ulrich [26] | 2005 | American | CRC | PB | 1600/1962 | 64.9/65.0 | 56.0/53.0 | Taqman | 0.13 |
| Yang [27] | 2005 | Japanese | EC | HB | 165/494 | 61.4/61.4 | 89.7/89.7 | RFLP | 0.43 |
| Wang [28] | 2006 | Chinese | PC | HB | 101/337 | NA/NA | 64.4/65.6 | RFLP | 0.86 |
| Koushik [29] | 2006 | American | CRC | PB | 363/804 | 68.2/68.0 | 47.6/42.0 | Taqman | 0.18 |
| Chen [30] | 2006 | Chinese | CRC | PB | 199/413 | 62.5/61.9 | 50.8/51.7 | RFLP | 0.18 |
| Curtin [31] | 2007 | American | CRC | PB | 916/1974 | NA/NA | NA/NA | Taqman | 0.09 |
| Zhang [32] | 2007 | Polish | GC | PB | 293/413 | 63.0/63.7 | 66.2/64.6 | Taqman | 0.22 |
| Theodoratou [33] | 2008 | Scottish | CRC | PB | 999/1010 | 62.3/62.7 | 57.3/56.9 | Array | 0.27 |
| Guerreiro [34] | 2008 | Portuguese | CRC | PB | 196/200 | 64.2/62.2 | 53.1/53.0 | Taqman | 0.41 |
| Suzuki [35] | 2008 | Japanese | PC | HB | 157/783 | NA/NA | 71.3/71.3 | Taqman | 0.56 |
| Zhang [36] | 2008 | Chinese | CRC | HB | 298/300 | 57.7/57.6 | 56.3/56.7 | RFLP | 0.13 |
| Ott [37] | 2008 | German | EC, GC | HB | 588/245 | 59.7/39 | 70.0/76.7 | RFLP | 0.97 |
| Steck [38] | 2008 | American | CRC | PB | 546/855 | 63.8/65.9 | NA/NA | Taqman | 0.14 |
| de Vogel [39] | 2009 | Dutch | CRC | PB | 696/1805 | NA/NA | 55.0/50.2 | SNaPShot | 0.31 |
| Zhang [40] | 2009 | Chinese | CRC | HB | 476/835 | 54.3/52.0 | 57.1/55.1 | RFLP | 0.67 |
| Eussen [41] | 2010 | European | CRC | PB | 1329/2364 | 58.9/58.7 | 51.0/53.0 | Mass spectrometry | 0.52 |
| Levine [42] | 2010 | American | CRC | PB | 1806/2879 | 53.5/54.0 | 51.3/44.4 | iPLEX | 0.17 |
| Eussen [43] | 2010 | European | GC | PB | 243/616 | 58.9/58.7 | 41.0/41.0 | Mass spectrometry | 0.12 |
| Jokić [44] | 2011 | Croatian | CRC | PB | 300/300 | 62.2/61.4 | 54.0/50.6 | Taqman | 0.82 |
| Guimarães [45] | 2011 | Brazilian | CRC | PB | 113/188 | 59.0/54.0 | 53.1/64.4 | RFLP | 0.06 |
| Kim [46] | 2011 | Korean | CRC | HB | 67/53 | 61.8/58.7 | 52.2/43.4 | RFLP | 0.12 |
| Cui [47] | 2012 | Chinese | HCC | PB | 356/641 | 56.6/58.7 | 83.1/43.5 | RFLP | 0.92 |
| Martinelli [48] | 2012 | Italian | CRC | PB | 71/80 | 69.0/58.0 | 59.2/53.8 | RFLP | 0.21 |
NA: not available, HB: hospital-based, PB: population-based, CRC: colorectal cancer, EC: esophagus cancer, HCC: hepatocellular carcinoma, GC: gastric cancer, PC: pancreatic cancer.
Quantitative Data Synthesis
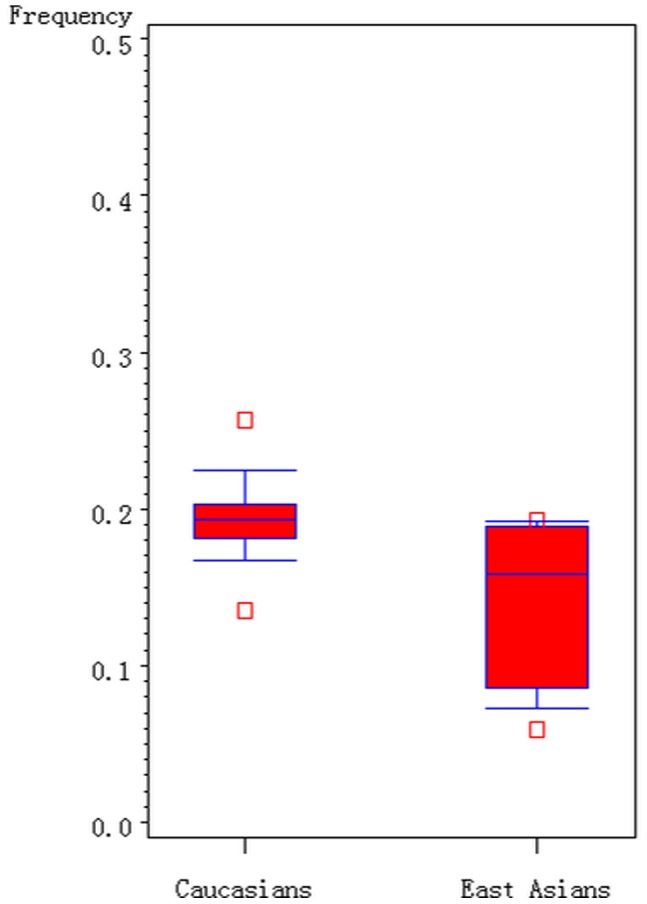
As shown in Figure 2, the G allele distribution of the A2756G polymorphism varies among the controls across different ethnicities, ranging from 0.06 to 0.25. For East Asian controls, the G allele frequency was 0.14 (95% CI: 0.11–0.18), which was lower than that in Caucasian controls (0.20; 95% CI: 0.18–0.22), indicating a significant difference among East Asians as compared with Caucasians (P = 0.003).
Figure 2. Frequencies of the G allele of MTR A2756G polymorphism among controls stratified by ethnicity.
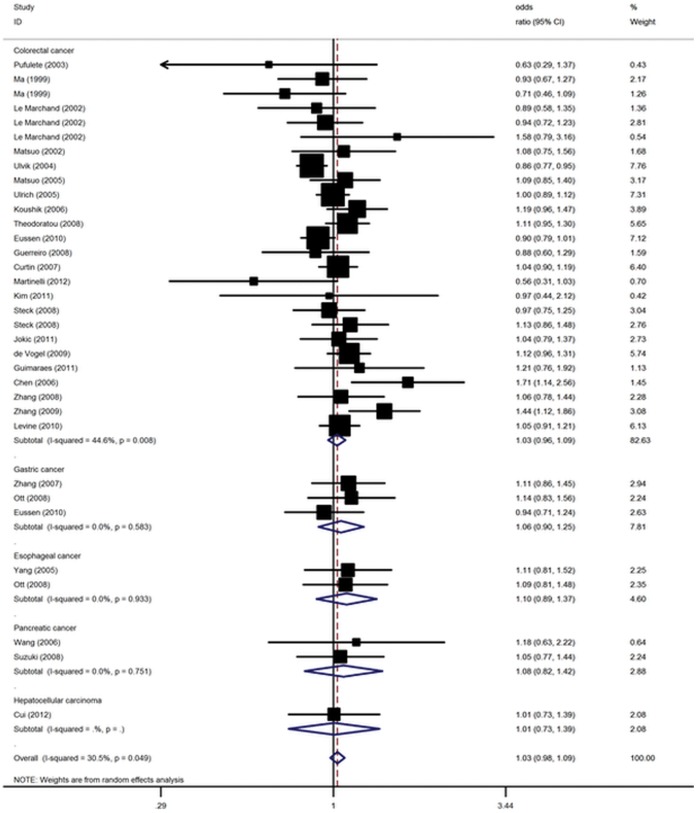
Overall, there was no evidence of an association between the increased risk of digestive system cancer and the A2756G polymorphism in different genetic models when all eligible studies were pooled into the meta-analysis. Under random effect model, the per-allele overall OR of the G variant for digestive system cancer was 1.03 [95% CI: 0.98–1.09, P(Z) = 0.25, P(Q) = 0.05], with corresponding results under dominant and recessive genetic models of 1.03 [95% CI: 0.97–1.10, P(Z) = 0.33, P(Q) = 0.06] and 1.02 [95% CI: 0.89–1.17, P(Z) = 0.79, P(Q) = 0.25], respectively.
This analysis is based on pooling of data from a number of different ethnic populations. When stratifying for ethnicity, an OR of 1.00 (95% CI: 0.94–1.05, P = 0.88) and 1.13 (95% CI: 1.02–1.25, P = 0.02) resulted for G allele, among Caucasians and East Asians, respectively. In the stratified analysis by cancer type, no significantly increased cancer risks were found for colorectal cancer, esophagus cancer, gastric cancer, pancreatic cancer and hepatocellular carcinoma in all genetic models (Figure 3). By considering control source subgroups, the OR was 1.01 (95% CI: 0.95–1.07, P = 0.78) in population-based controls, compared to 1.13 (95% CI: 1.02–1.25, P = 0.02) in hospital controls. In addition, no significant association between genotype of MTR A2756G and digestive system cancer risk in the stratified analysis according to sample size or genotyping method (Table 2).
Figure 3. Forest plot from the meta-analysis of digestive system cancer and MTR A2756G polymorphism under random effect model.
Table 2. Main results of pooled odds ratios (ORs) with confidence interval (CI) in the meta-analysis.
| Sub-group analysis | No. ofdatasets | No. ofcases/controls | G Allele | Dominant Model | Recessive Model | ||||||
| OR (95% CI) | P(Z) | P(Q) | OR (95% CI) | P(Z) | P(Q) | OR (95% CI) | P(Z) | P(Q) | |||
| Overall | 34 | 15368/23959 | 1.03 (0.98–1.09) | 0.25 | 0.05 | 1.03 (0.97–1.10) | 0.33 | 0.06 | 1.02 (0.89–1.17) | 0.79 | 0.25 |
| Cancer type | |||||||||||
| Colorectal cancer (Overall) | 26 | 13465/20430 | 1.03 (0.96–1.09) | 0.25 | 0.008 | 1.03 (0.96–1.12) | 0.39 | 0.01 | 0.99 (0.85–1.15) | 0.86 | 0.23 |
| Colorectal cancer (Caucasians ) | 17 | 11396/17014 | 0.98 (0.91–1.05) | 0.56 | 0.05 | 0.98 (0.91–1.06) | 0.68 | 0.11 | 0.93 (0.91–1.06) | 0.45 | 0.12 |
| Colorectal cancer (Asians) | 7 | 1754/3007 | 1.17 (1.00–1.36) | 0.06 | 0.14 | 1.19 (0.98–1.45) | 0.08 | 0.08 | 1.21 (0.84–1.77) | 0.31 | 0.75 |
| Gastric cancer | 3 | 806/1029 | 1.06 (0.90–1.25) | 0.50 | 0.58 | 1.04 (0.86–1.25) | 0.72 | 0.42 | 1.30 (0.81–2.07) | 0.27 | 0.73 |
| Esophagus cancer | 2 | 483/739 | 1.10 (0.89–1.37) | 0.38 | 0.93 | 1.15 (0.89–1.48) | 0.29 | 0.90 | 0.97 (0.48–1.93) | 0.92 | 0.52 |
| Pancreatic cancer | 2 | 258/1120 | 1.08 (0.82–1.42) | 0.60 | 0.75 | 1.01 (0.73–1.40) | 0.95 | 0.81 | 2.80 (0.40–19.62) | 0.30 | 0.10 |
| Hepatocellular carcinoma | 1 | 356/641 | 1.01 (0.73–1.39) | 0.97 | NA | 0.96 (0.68–1.37) | 0.83 | NA | 1.81 (0.52–6.30) | 0.35 | NA |
| Ethnicity | |||||||||||
| Caucasian | 21 | 12520/18288 | 1.00 (0.94–1.05) | 0.88 | 0.10 | 0.99 (0.93–1.06) | 0.87 | 0.16 | 0.96 (0.82–1.13) | 0.62 | 0.18 |
| East Asian | 11 | 2533/5262 | 1.13 (1.02–1.25) | 0.02 | 0.39 | 1.13 (0.99–1.29) | 0.06 | 0.22 | 1.31 (0.96–1.79) | 0.09 | 0.72 |
| Other | 2 | 315/409 | 1.18 (0.92–1.52) | 0.20 | 0.37 | 1.18 (0.87–1.60) | 0.30 | 0.39 | 1.53 (0.76–3.05) | 0.23 | 0.72 |
| Sample size | |||||||||||
| <500 | 27 | 5854/9773 | 1.07 (1.00–1.14) | 0.05 | 0.34 | 1.07 (0.99–1.16) | 0.08 | 0.34 | 1.14 (0.95–1.38) | 0.17 | 0.87 |
| ≥500 | 7 | 9514/14186 | 0.99 (0.92–1.08) | 0.90 | 0.02 | 0.99 (0.90–1.09) | 0.84 | 0.03 | 0.95 (0.73–1.25) | 0.74 | 0.008 |
| Source of control | |||||||||||
| Population | 23 | 13089/19824 | 1.01 (0.95–1.07) | 0.78 | 0.03 | 1.01 (0.94–1.08) | 0.83 | 0.04 | 0.98 (0.85–1.14) | 0.83 | 0.24 |
| Hospital | 11 | 2279/4135 | 1.13 (1.02–1.25) | 0.02 | 0.77 | 1.14 (1.01–1.28) | 0.03 | 0.73 | 1.28 (0.93–1.75) | 0.13 | 0.54 |
| Genotyping method | |||||||||||
| RFLP | 19 | 3756/5802 | 1.07 (0.97–1.18) | 0.13 | 0.20 | 1.08 (0.97–1.21) | 0.16 | 0.18 | 1.08 (0.83–1.40) | 0.57 | 0.59 |
| Others | 15 | 11612/18157 | 1.01 (0.95–1.07) | 0.12 | 0.77 | 1.00 (0.94–1.07) | 0.94 | 0.15 | 1.03 (0.86–1.23) | 0.76 | 0.09 |
Although the formal test for heterogeneity was not significant in the overall analysis, we conducted meta-regression as there were also grounds for considering the ethnicity, sample size, genotyping method, cancer type, and clinical characteristics of cases and controls (age, and sex distribution) as potential sources of heterogeneity. In meta-regression analysis, ethnicity (P = 0.19), cancer type (P = 0.96), source of controls (P = 0.07), mean age of cases (P = 0.62) and controls (P = 0.72), genotyping method (P = 0.29) and gender distribution among cases (P = 0.65) and controls (P = 0.97) were not significantly correlated with the magnitude of the genetic effect. By contrast, the sample size (P = 0.008) was significantly correlated with between-study heterogeneity.
Sensitivity Analyses and Publication Bias
In order to assess the stability of the results of the meta-analysis, we performed a sensitivity analysis through sequentially excluded individual studies. Statistically similar results were obtained after sequentially excluding each study, suggesting stability of the meta-analyses. Begg’s funnel plot and Egger’s test were performed to access the publication bias of the literatures. The shape of the funnel plots was symmetrical for the polymorphism (Figure S1). The statistic results also indicated a lack of publication bias of the current meta-analysis (Egger’s test: P = 0.25).
Discussion
Large sample and unbiased epidemiological studies of predisposition genes polymorphisms could provide insight into the in vivo relationship between candidate genes and diseases. Methionine synthesis is the first step in the synthesis of SAM which is a universal methyl-group donor involved in methylation reactions including DNA methylation. This report is the first meta-analysis examining the effect of MTR A2756G polymorphism on the risk of digestive system cancer. In total, the meta-analysis involved 29 studies for digestive system cancer which provided 15,368 cases and 23,959 controls. Our results demonstrated that the G allele of the A2756G polymorphism on MTR is not a risk factor for developing digestive system cancer. Sensitivity analysis indicated robustness of our results.
In meta-analysis, heterogeneity evaluation was always conducted. Thus, subgroup meta-analyses were performed. In cancer type subgroups, no statistically significant association between MTR polymorphism and different types of cancer were found. However, in our meta-analysis, only one or two studies were available for some specific cancers, and they had limited sample size, and hence the results may be capricious and should be interpreted with caution. Meta-analysis is often dominated by a few large studies, which markedly reduces the evidence from smaller studies. However, in the stratified analysis according to sample size, no significant association between digestive system cancer susceptibility an MTR were found both in large and small studies for all genetic models. Besides, studies using different genotyping method also get consistent negative results.
In the stratified analysis by ethnicity, no significant associations were found in Caucasians for the polymorphism in all genetic models. However, we observed a marginal significant association between A2756G polymorphism and increased risk for digestive system cancer in East Asian populations. There are several explanations of this phenomenon. First, cancer is a complex disease and different genetic backgrounds may cause the discrepancy since the G allele distributions of the A2756G polymorphism varies between East Asian and Caucasian. In addition, different populations usually have different linkage disequilibrium patterns. A polymorphism may be in close linkage with another nearby causal variant in one ethnic population but not in another. MTR A2756G polymorphism may be in close linkage with different nearby causal variants in different populations. Moreover, clinical heterogeneity like age, sex ratio, dietary, years from onset and disease severity may also explain the discrepancy. Different populations may have differences in dietary intake of nutrients, some of which take part in the tumor formation.
Our results indicated that marginal significantly increased digestive system cancer risk in G allele carriers were found among the hospital-based studies but not in population-based studies. This reason may be that the hospital-based studies have some biases because such controls may just represent a sample of ill-defined reference population, and may not be representative of the general population very well, particularly when the genotypes under investigation were associated with the disease conditions that the hospital-based controls may have. Therefore, using a proper and representative population-based control subjects is very important to reduce biases in such genetic association studies.
Digestive system cancer is an extremely complex disease and the same polymorphism may have different roles in different tumor sites. Therefore, more studies for a specific type of digestive system cancer are needed to identify potential tumor-specific effect of MTR polymorphism. In addition, it is possible that the effect of a single polymorphism on digestive system cancer risk may be very small. Several other polymorphisms were identified, suggesting that possible combined effects of these polymorphisms on MTR activity may exist [49]. Furthermore, the effect of single genetic factor on the risk of digestive system cancer may be more pronounced in the presence of other common genetic or environmental risk factors such as alcohol abuse, smoking, hepatitis virus infection.
Compared with the previous meta-analysis [50], [51], the present study is much larger, with more than twice as many digestive system cancer cases as the earlier meta-analysis. In addition, several subgroup analysis and meta-regression analysis were conducted to identify potential source of heterogeneity.
Some limitations of this meta-analysis should be acknowledged. Firstly, the subgroup meta-analyses considering interactions between MTR genotype and different tumor type are based on a small number of studies with such information available. Secondly, our results were based on unadjusted estimates, while a more precise analysis should be conducted if individual-level data were available, which would allow for the adjustment by other covariates including drinking status, cigarette consumption, folate and vitamin B12 intake, family history, environmental factors and lifestyle [52]. Thirdly, only published studies were included in this meta-analysis. Therefore, publication bias may have occurred, even though the use of a statistical test did not show it. In spite of these, our present meta-analysis also had some advantages. First, no significant between studies heterogeneity were detected in all comparison. Second, no publication biases were found, indicating that the whole pooled results may be unbiased.
To conclude, our meta-analysis did not support an association of the A2756G polymorphism of MTR with digestive system cancer. For future association studies, well-designed studies with large sample size in diverse ethnic populations, more types of digestive system cancers along with tissue-specific biochemical, functional and expressional characteristics are required.
Supporting Information
Begg’s funnel plot of MTR A2756G polymorphism and digestive system cancer.
(TIF)
(DOC)
Funding Statement
This work was supported by a grant from National Natural Science Foundation of China (81201535), Shanghai Natural Science Foundation (12ZR1436000), Knowledge Innovation Program of Shanghai Institutes for Biological Sciences (2012KIP203) and Youth Innovation Promotion Association, Chinese Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 2. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46: 765–81. [DOI] [PubMed] [Google Scholar]
- 4. Jemal A (2010) Center MM, DeSantis C, Ward EM (2010) Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 19: 1893–907. [DOI] [PubMed] [Google Scholar]
- 5. Hitt NP, Hendryx M (2010) Ecological integrity of streams related to human cancer mortality rates. Ecohealth 7: 91–104. [DOI] [PubMed] [Google Scholar]
- 6. Robertson KD, Wolffe AP (2000) DNA methylation in health and disease. Nat Rev Genet 1: 11–19. [DOI] [PubMed] [Google Scholar]
- 7. Jones PA, Laird PW (1999) Cancer epigenetics comes of age. Nat Genet 21: 163–167. [DOI] [PubMed] [Google Scholar]
- 8. Esteller M, Tortola S, Toyota M, Capella G, Peinado MA, et al. (2000) Hypermethylation-associated inactivation of p14(ARF) is independent of p16(INK4a) methylation and p53 mutational status. Cancer Res 60: 129–133. [PubMed] [Google Scholar]
- 9. Waki T, Tamura G, Tsuchiya T, Sato K, Nishizuka S, et al. (2002) Promoter methylation status of E-cadherin, hMLH1, and p16 genes in non-neoplastic gastric epithelia. Am J Pathol 161: 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banerjee RV, Matthews RG (1990) Cobalamin-dependent methionine synthase. FASEB J 4: 1450–1459. [DOI] [PubMed] [Google Scholar]
- 11. Leclerc D, Campeau E, Goyette P, Adjalla CE, Christensen B, et al. (1996) Human methionine synthase: cDNA cloning and identification of mutations in patients of the cblG complementation group of folate/cobalamin disorders. Hum Mol Genet 5: 1867–1874. [DOI] [PubMed] [Google Scholar]
- 12. Goode EL, Potter JD, Bigler J, Ulrich CM (2004) Methionine synthase D919G polymorphism, folate metabolism, and colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev 13: 157–162. [DOI] [PubMed] [Google Scholar]
- 13. Morita H, Kurihara H, Sugiyama T, Hamada C, Kurihara Y, et al. (1999) Polymorphism of the methionine synthase gene : association with homocysteine metabolism and late-onset vascular diseases in the Japanese population. Arterioscler Thromb Vasc Biol 19: 298–302. [DOI] [PubMed] [Google Scholar]
- 14. Huang L, Song XM, Zhu WL, Li Y (2008) Plasma homocysteine and gene polymorphisms associated with the risk of hyperlipidemia in northern Chinese subjects. Biomed Environ Sci 21: 514–520. [DOI] [PubMed] [Google Scholar]
- 15. Ma J, Stampfer MJ, Christensen B, Giovannucci E, Hunter DJ, et al. (1999) A polymorphism of the methionine synthase gene: association with plasma folate, vitamin B12, homocyst(e)ine, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev 8: 825–829. [PubMed] [Google Scholar]
- 16. Paz MF, Avila S, Fraga MF, Pollan M, Capella G, et al. (2002) Germ-line variants in methyl-group metabolism genes and susceptibility to DNA methylation in normal tissues and human primary tumors. Cancer Res 62: 4519–4524. [PubMed] [Google Scholar]
- 17. Lau J, Ioannidis JP, Schmid CH (1997) Quantitative synthesis in systematic reviews. Ann Intern Med 127: 820–826. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19. Thompson SG, Sharp SJ (1999) Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 18: 2693–2708. [DOI] [PubMed] [Google Scholar]
- 20. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Le Marchand L, Donlon T, Hankin JH, Kolonel LN, Wilkens LR, et al. (2002) B-vitamin intake, metabolic genes, and colorectal cancer risk (United States). Cancer Causes Control 13: 239–248. [DOI] [PubMed] [Google Scholar]
- 22. Matsuo K, Hamajima N, Hirai T, Kato T, Inoue M, et al. (2002) Methionine Synthase Reductase Gene A66G Polymorphism is Associated with Risk of Colorectal Cancer. Asian Pac J Cancer Prev 3: 353–359. [PubMed] [Google Scholar]
- 23. Pufulete M, Al-Ghnaniem R, Leather AJ, Appleby P, Gout S, et al. (2003) Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology 124: 1240–1248. [DOI] [PubMed] [Google Scholar]
- 24. Ulvik A, Vollset SE, Hansen S, Gislefoss R, Jellum E, et al. (2004) Colorectal cancer and the methylenetetrahydrofolate reductase 677C -> T and methionine synthase 2756A -> G polymorphisms: a study of 2,168 case-control pairs from the JANUS cohort. Cancer Epidemiol Biomarkers Prev 13: 2175–2180. [PubMed] [Google Scholar]
- 25. Matsuo K, Ito H, Wakai K, Hirose K, Saito T, et al. (2005) One-carbon metabolism related gene polymorphisms interact with alcohol drinking to influence the risk of colorectal cancer in Japan. Carcinogenesis 26: 2164–2171. [DOI] [PubMed] [Google Scholar]
- 26. Ulrich CM, Curtin K, Potter JD, Bigler J, Caan B, et al. (2005) Polymorphisms in the reduced folate carrier, thymidylate synthase, or methionine synthase and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 14: 2509–2516. [DOI] [PubMed] [Google Scholar]
- 27. Yang CX, Matsuo K, Ito H, Shinoda M, Hatooka S, et al. (2005) Gene-environment interactions between alcohol drinking and the MTHFR C677T polymorphism impact on esophageal cancer risk: results of a case-control study in Japan. Carcinogenesis 26: 1285–1290. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Lin DX, Lu XH, Miao XP, Li H (2006) Study on the relations between genetic polymorphisms in methylenetetrahydrofolate reductase, methionine synthase and the risk of pancreatic cancer. Zhonghua Liu Xing Bing Xue Za Zhi 27: 50–54. [PubMed] [Google Scholar]
- 29. Koushik A, Kraft P, Fuchs CS, Hankinson SE, Willett WC, et al. (2006) Nonsynonymous polymorphisms in genes in the one-carbon metabolism pathway and associations with colorectal cancer. Cancer Epidemiol Biomarkers Prev 15: 2408–2417. [DOI] [PubMed] [Google Scholar]
- 30. Chen K, Song L, Jin MJ, Fan CH, Jiang QT, et al. (2006) Association between genetic polymorphisms in folate metabolic enzyme genes and colorectal cancer: a nested case-control study. Zhonghua Zhong Liu Za Zhi 28: 429–432. [PubMed] [Google Scholar]
- 31. Curtin K, Slattery ML, Ulrich CM, Bigler J, Levin TR, et al. (2007) Genetic polymorphisms in one-carbon metabolism: associations with CpG island methylator phenotype (CIMP) in colon cancer and the modifying effects of diet. Carcinogenesis 28: 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang FF, Terry MB, Hou L, Chen J, Lissowska J, et al. (2007) Genetic polymorphisms in folate metabolism and the risk of stomach cancer. Cancer Epidemiol Biomarkers Prev 16: 115–121. [DOI] [PubMed] [Google Scholar]
- 33. Theodoratou E, Farrington SM, Tenesa A, McNeill G, Cetnarskyj R, et al. (2008) Dietary vitamin B6 intake and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 17: 171–182. [DOI] [PubMed] [Google Scholar]
- 34. Guerreiro CS, Carmona B, Gonçalves S, Carolino E, Fidalgo P, et al. (2008) Risk of colorectal cancer associated with the C677T polymorphism in 5,10-methylenetetrahydrofolate reductase in Portuguese patients depends on the intake of methyl-donor nutrients. Am J Clin Nutr 88: 1413–1418. [DOI] [PubMed] [Google Scholar]
- 35. Suzuki T, Matsuo K, Sawaki A, Mizuno N, Hiraki A, et al. (2008) Alcohol Drinking and One-Carbon Metabolism-Related Gene Polymorphisms on Pancreatic Cancer Risk. Cancer Epidemiol Biomarkers Prev 17: 2742–2747. [DOI] [PubMed] [Google Scholar]
- 36. Zhang YL, Yuan XY, Zhang Z, Yang H, Zhou YH, et al. (2008) Relationship of genetic polymorphisms in methylenetetrahydrofoIate reductase and alcohol drinking with the risk of colorectal cancer. Zhonghua Zhong Liu Fang Zhi Za Zhi 15: 1298–1301. [Google Scholar]
- 37. Ott N, Geddert H, Sarbia M (2008) Polymorphisms in methionine synthase (A2756G) and cystathionine beta-synthase (844ins68) and susceptibility to carcinomas of the upper gastrointestinal tract. J Cancer Res Clin Oncol 134: 405–410. [DOI] [PubMed] [Google Scholar]
- 38. Steck SE, Keku T, Butler LM, Galanko J, Massa B, et al. (2008) Polymorphisms in Methionine Synthase, Methionine Synthase Reductase and Serine Hydroxymethyltransferase, Folate and Alcohol Intake, and Colon Cancer Risk. J Nutrigenet Nutrigenomics 1: 196–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Vogel S, Wouters KA, Gottschalk RW, van Schooten FJ, de Goeij AF, et al. (2009) Genetic variants of methyl metabolizing enzymes and epigenetic regulators: associations with promoter CpG island hypermethylation in colorectal cancer. Cancer Epidemiol Biomarkers Prev 18: 3086–3096. [DOI] [PubMed] [Google Scholar]
- 40. Zhang YL, Yuan XY, Zhang Z, Yang H, Zhou YH, et al. (2009) Relationship of genetic polymorphisms in thymidylate synthase and alcohol drinking with risk of colorectal cancer. Zhonghua Zhong Liu Fang Zhi Za Zhi 16: 568–572. [Google Scholar]
- 41. Eussen SJ, Vollset SE, Igland J, Meyer K, Fredriksen A, et al. (2010) Plasma Folate, Related Genetic Variants, and Colorectal Cancer Risk in EPIC. Cancer Epidemiol Biomarkers Prev 19: 1328–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levine AJ, Figueiredo JC, Lee W, Conti DV, Kennedy K, et al. (2010) A Candidate Gene Study of Folate-Associated One Carbon Metabolism Genes and Colorectal Cancer Risk. Cancer Epidemiol Biomarkers Prev 19: 1812–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eussen SJ, Vollset SE, Hustad S, Midttun Ø, Meyer K, et al. (2010) Vitamins B2 and B6 and genetic polymorphisms related to one-carbon metabolism as risk factors for gastric adenocarcinoma in the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 19: 28–38. [DOI] [PubMed] [Google Scholar]
- 44. Jokić M, Brčić-Kostić K, Stefulj J, Catela Ivković T, Božo L, et al. (2011) Association of MTHFR, MTR, MTRR, RFC1, and DHFR Gene Polymorphisms with Susceptibility to Sporadic Colon Cancer. DNA Cell Biol 30: 771–776. [DOI] [PubMed] [Google Scholar]
- 45. Guimarães JL, Ayrizono Mde L, Coy CS, Lima CS (2011) Gene polymorphisms involved in folate and methionine metabolism and increased risk of sporadic colorectal adenocarcinoma. Tumour Biol 32: 853–861. [DOI] [PubMed] [Google Scholar]
- 46. Kim JW, Park HM, Choi YK, Chong SY, Oh D, Kim NK (2011) Polymorphisms in genes involved in folate metabolism and plasma DNA methylation in colorectal cancer patients. Oncol Rep 25: 167–172. [PubMed] [Google Scholar]
- 47. Cui LH, Song Y, Si H, Shen F, Shin MH, et al. (2012) Folate metabolism-related gene polymorphisms and susceptibility to primary liver cancer in North China. Med Oncol 29: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 48. Martinelli M, Scapoli L, Mattei G, Ugolini G, Montroni I, et al. (2012) A candidate gene study of one-carbon metabolism pathway genes and colorectal cancer risk. Br J Nutr 16: 1–6. [DOI] [PubMed] [Google Scholar]
- 49. Al Farra HY (2010) Methionine synthase polymorphisms (MTR 2756 A>G and MTR 2758 C>G) frequencies and distribution in the Jordanian population and their correlation with neural tube defects in the population of the northern part of Jordan. Indian J Hum Genet 16: 138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou D, Mei Q, Luo H, Tang B, Yu P (2012) The polymorphisms in methylenetetrahydrofolate reductase, methionine synthase, methionine synthase reductase, and the risk of colorectal cancer. Int J Biol Sci 8: 819–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu K, Zhang J, Zhang J, Dou C, Gu S, et al. (2010) Methionine synthase A2756G polymorphism and cancer risk: a meta-analysis. Eur J Hum Genet 8: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rodriguez C, Calle EE, Miracle-McMahill HL, Tatham LM, Wingo PA, et al. (1997) Family history and risk of fatal prostate cancer. Epidemiology 8: 653–659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Begg’s funnel plot of MTR A2756G polymorphism and digestive system cancer.
(TIF)
(DOC)