Abstract
Background and Objectives
We sought to determine whether high-dose aspirin is necessary for the acute therapy of Kawasaki disease (KD) in the intravenous immunoglobulin (IVIG) era.
Subjects and Methods
Two groups of KD patients treated during the different periods were included. Study group (n=51, treated with IVIG without concomitant use of aspirin in the acute phase) was compared with control group (n=129, treated with IVIG plus high-dose aspirin) with regard to the response to IVIG, duration of fever after IVIG completion, time to C-reactive protein (CRP) <3 mg/dL, and the incidence of coronary artery lesions (CALs).
Results
There was no difference between the groups in age, sex, and duration of fever before treatment. Pre-IVIG laboratory measures also did not differ from each other. IVIG-resistant cases were 8 (15.7%) in study group and 22 (17.1%) in control group (p=1.000). Mean duration of fever after IVIG completion in IVIG-responsive patients was 13.3±13.5 hours in study group compared to 6.2±8.3 hours in control group (p=0.000). The mean time to decrease in CRP was 4.0±1.7 days in study group and 4.1±2.2 days in control group (p=0.828). There were 2 (3.9%) patients with CALs in study group and 10 (7.8%) in control group (p=0.514).
Conclusion
Although high-dose aspirin shortens the duration of fever, treatment without aspirin in the acute phase has no influence on the response to IVIG, resolution of inflammation, or the development of CALs. In the IVIG era, high-dose aspirin may provide little benefit to the treatment in the acute phase of KD.
Keywords: Kawasaki disease, Aspirin, Therapeutics
Introduction
Although aspirin remains an integral component of standard therapy for acute Kawasaki disease (KD) in the intravenous immunoglobulin (IVIG) era, the optimal dose remains controversial. The American Heart Association currently recommends high-dose aspirin (80 to 100 mg/kg/day) during the acute phase,1) while moderate-dose aspirin (30 to 50 mg/kg/day) is used in Japan due to concern about hepatic toxicity.2) Gastritis, upper gastrointestinal bleeding,3) sensorineural hearing loss,4) and rarely Reye syndrome5) are also concerns with high-dose aspirin in children with KD. The therapeutic steady state salicylate levels are rarely achieved during the acute phase of KD because of decreased absorption6) and increased renal clearance of aspirin.7) Despite this, hypoalbuminemia in the acute phase of the illness promotes less protein binding and higher free drug levels.8) Meanwhile, the primary goal of treating children with KD is to prevent coronary artery lesions (CALs). Some reports9),10) have shown that the incidence of CALs is highly dependent on the dose of IVIG, but independent of aspirin dose. This suggests that only antiplatelet doses of aspirin may be adequate during the acute phase of the disease.
Thus, considering the risks of drug toxicity and the lack of evidence for prevention of CALs, the role of high-dose aspirin in the acute phase of KD needs to be reassessed. The purpose of this study was to evaluate the effect of treatment without high-dose aspirin in the acute phase of KD and thereby determine whether high-dose aspirin is necessary for the acute therapy of KD in the IVIG era.
Subjects and Methods
A total of 51 children who met the diagnostic criteria for KD1) between October 2010 and September 2011 were enrolled as the study group. Subjects comprised 30 (58.8%) male and 21 female, aged 2.5 months to 11.5 years. All patients were treated with IVIG (2 g/kg) as a single infusion over 10 to 12 hours without concomitant use of aspirin 2 to 20 days after the onset of the disease. After defervescence, low-dose aspirin (3 to 5 mg/kg) was given as a single daily dose. When the patients remained febrile after the first IVIG therapy, they were retreated with additional therapy without aspirin. As the control group, we included a total of 129 KD patients who were treated with IVIG (2 g/kg) plus high-dose aspirin (80 to 100 mg/kg/day) 2 to 9 days after the onset of the illness, and then low-dose aspirin after defervescence between November 2008 and September 2010. This control group of patients was reported previously.11)
We measured white blood cell (WBC), percentage of neutrophils in WBCs (% neutrophils), hematocrit, platelet counts, serum sodium, aspartate aminotransferase (AST), alanine aminotransferase (ALT), protein, albumin, N-terminal pro-brain natriuretic peptide (NT-proBNP), and C-reactive protein (CRP) before and 48 to 72 hours after IVIG treatment. Kobayashi score12) was utilized as a marker of the severity of the disease. We arbitrarily considered CRP <3 mg/dL as an acceptable criterion for resolution of inflammation. When CRP remained >3 mg/dL following treatment, it was measured again 2 days later. Coronary arteries were assessed by echocardiography performed at the time of diagnosis and discharge, and repeated at weeks 4 and 8 after treatment, and then as needed thereafter. CALs were diagnosed according to the criteria of the Japanese Ministry of Health and Welfare.13) Patients who presented with CAL before initial treatment were excluded from the study.
Basic patient characteristics and laboratory measures before IVIG therapy were compared between the study and control group. We compared the response to initial IVIG, duration of fever after completion of initial IVIG treatment, time to CRP <3 mg/dL after IVIG administration, and the incidence of CALs between the groups. This study was approved by the Institutional Review Board of the hospital (ECT 12-07A-21).
Definition
Duration of fever to IVIG initiation was defined as the time from the onset of the disease to the start of initial IVIG infusion. Duration of fever after IVIG completion was defined as the time from the end of IVIG infusion to defervescence (<37.5℃). IVIG-responsive patients were defined as patients who became afebrile within 48 to 72 hours after initial IVIG infusion. IVIG-resistant patients were defined as those who required additional therapy owing to persistent fever (≥38℃) at least 48 hours after the end of initial IVIG infusion.
Data analyses
Analyses were performed with Statistical Package for the Social Sciences (SPSS) 12.0 statistical analysis software (SPSS Inc., Chicago, IL, USA). Data are presented as mean±SD or absolute numbers and percentages. Continuous variables were compared between the groups using Student t-test. Categorical variables were compared using either χ2 test or Fisher's exact test. A level of p<0.05 was considered statistically significant.
Results
Comparison in baseline patient demographics between the study and the control group
Patients treated with IVIG without concomitant high-dose aspirin did not differ from those treated with IVIG plus high-dose aspirin with respect to age, sex, duration of fever to IVIG initiation, and Kobayashi score (Table 1).
Table 1.
Comparison in basic characteristics between the study and control group
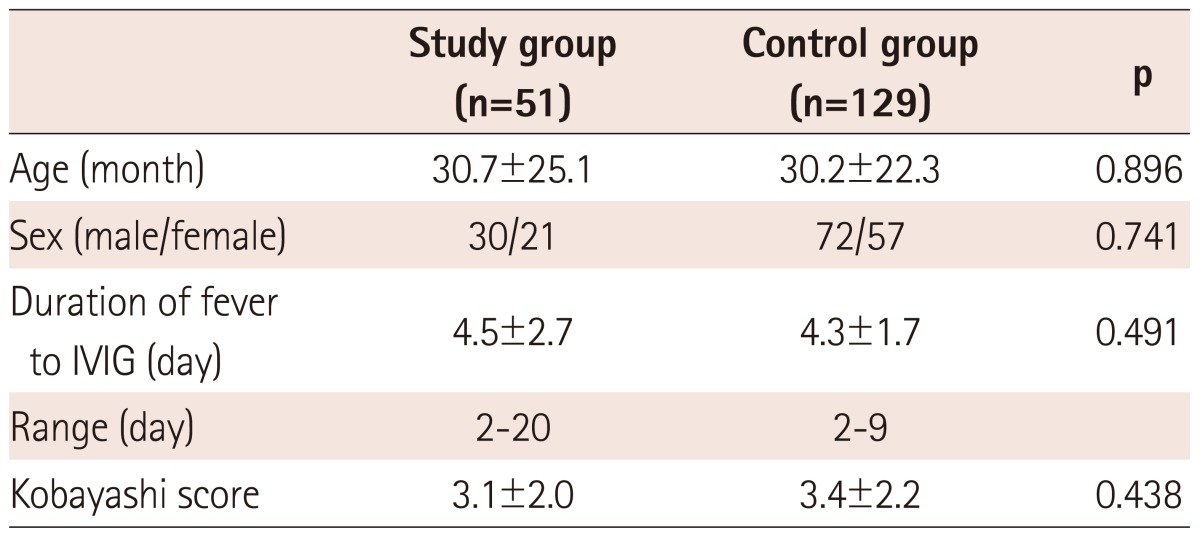
IVIG: intravenous immunoglobulin
Comparison in baseline laboratory measures between the study and the control group
There was no difference between the groups in WBC, % neutrophils, hematocrit, platelet counts, serum sodium, AST, ALT, protein, albumin, NT-proBNP, and CRP (Table 2).
Table 2.
Comparison in baseline laboratory measures between the study and control group
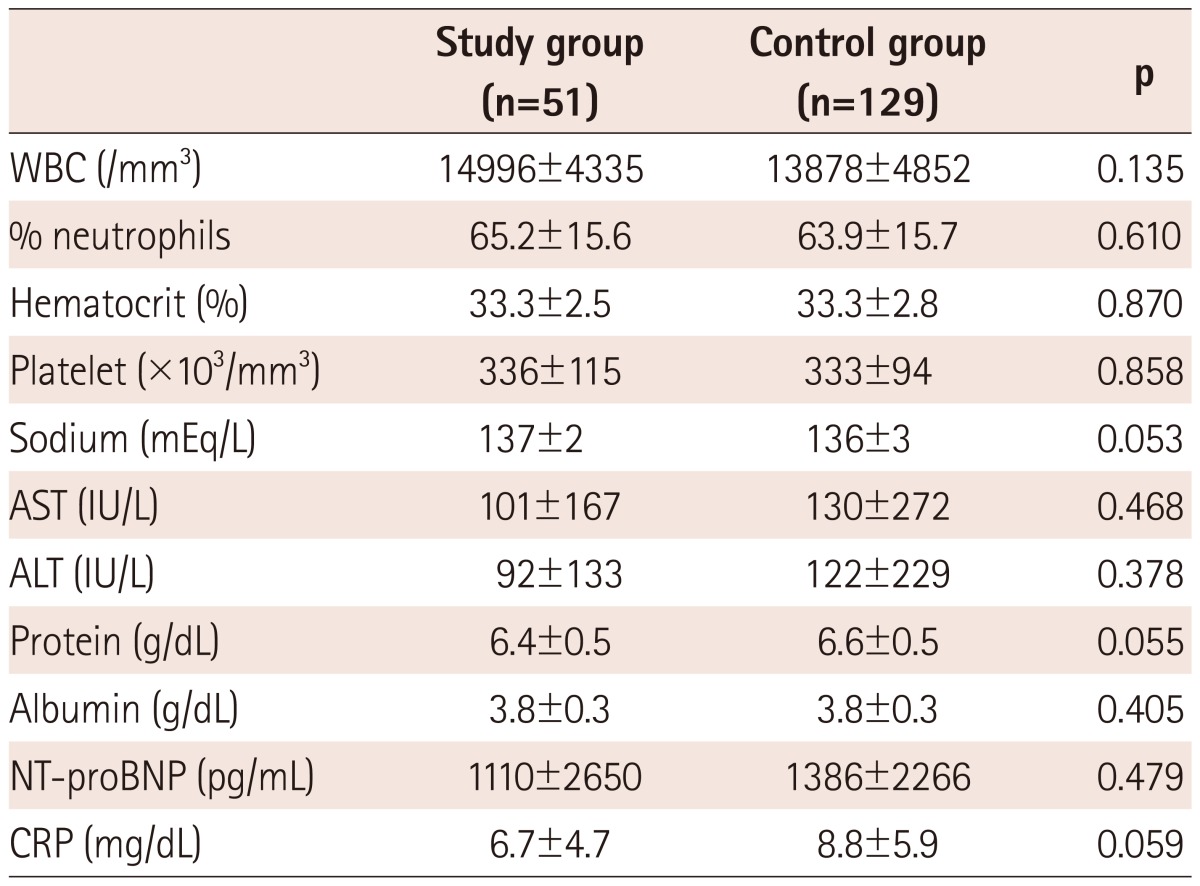
WBC: white blood cell, AST: aspartate aminotransferase, ALT: alanine aminotransferase, NT-proBNP: N-terminal pro-brain natriuretic peptide, CRP: C-reactive protein
Comparisons between the study and the control group in total patients
Response to initial intravenous immunoglobulin
Of a total of 51 patients treated with IVIG without aspirin, 43 (84.3%) patients completely responded to a single IVIG therapy, and 8 (15.7%) required retreatment: 7 patients had defervescence after a second dose of IVIG; and 1 received a second IVIG followed by infliximab therapy. Of a total of 129 patients treated with IVIG plus aspirin, 107 (82.9%) were IVIG-responsive patients, and 22 (17.1%) were IVIG-resistant patients. There was no difference in the response to IVIG between the study and control group (p=1.000) (Table 3).
Table 3.
Comparison in outcomes between the study and control group
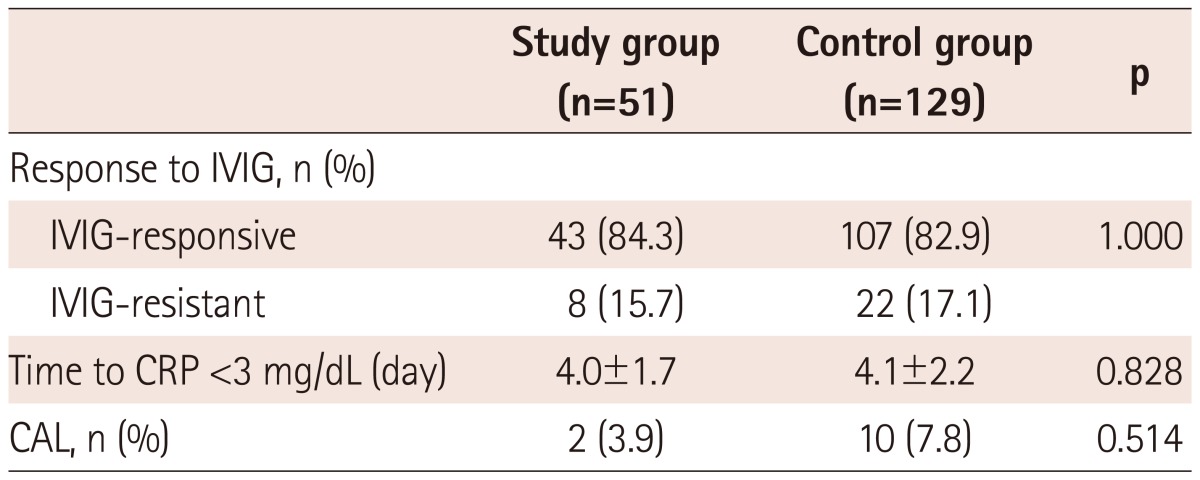
IVIG: intravenous immunoglobulin, CRP: C-reactive protein, CAL: coronary artery lesion
Time to decrease in C-reactive protein after initial intravenous immunoglobulin administration
The mean time to CRP <3 mg/dL was 4.0±1.7 days in the study group and 4.1±2.2 days in the control group, with no difference between the groups (p=0.828) (Table 3).
Incidence of coronary artery lesions
After IVIG therapy, CALs developed in 2 (3.9%) of the 51 patients in the study group and in 10 (7.8%) of the 129 patients in the control group. There was no significant difference in the incidence of CALs between the groups (p=0.514) (Table 3). All CALs were either small aneurysm or dilatation, all of which regressed to normal coronary artery at the 2-month follow-up.
Comparisons between the study and the control group in the intravenous immunoglobulin-responsive patients
Between the study and control group, no difference was found with respect to baseline patient demographics and laboratory measures in this group of patients (data not shown).
Duration of fever after completion of intravenous immunoglobulin treatment
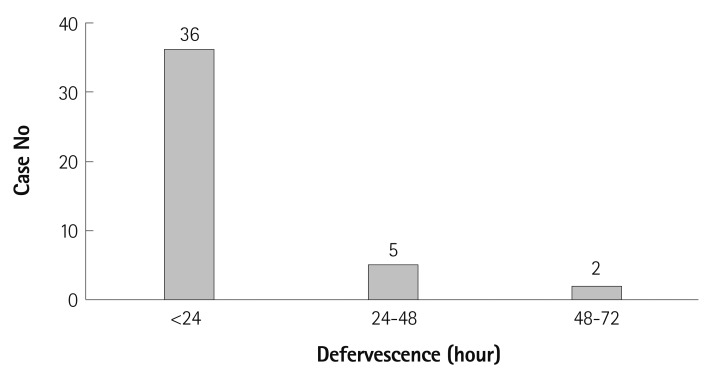
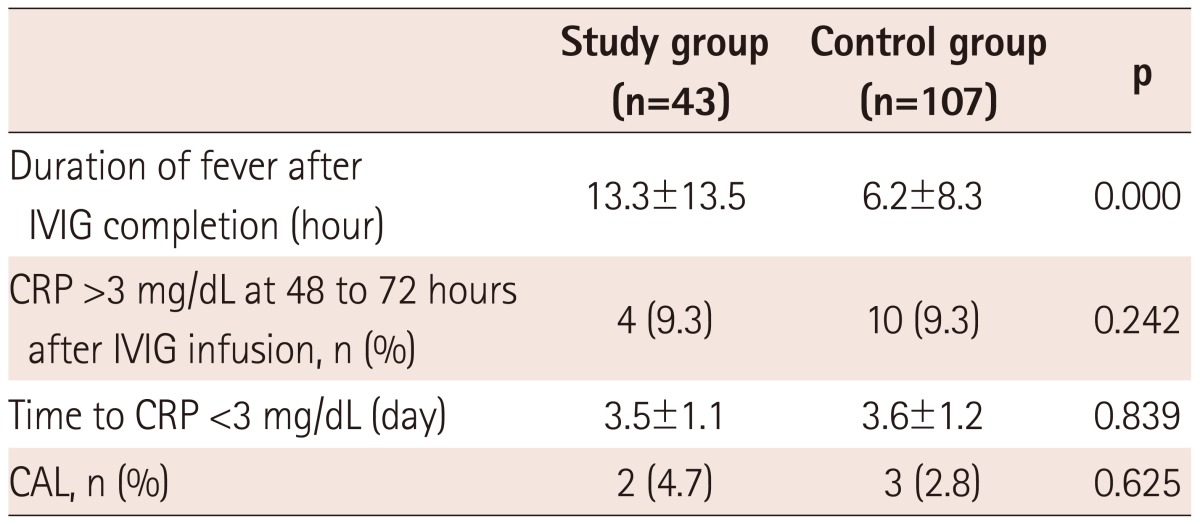
The mean duration of fever after IVIG completion was 13.3±13.5 hours in the study group compared to 6.2±8.3 hours in the control group (p=0.000) (Table 4). Of the 43 patients in the study group, 36 (83.7%) became afebrile within 24 hours (Fig. 1), while 98 (91.6%) of the 107 patients in the control group had defervescence within 24 hours after treatment.
Table 4.
Comparison in outcomes between the study and control group in the intravenous immunoglobulin-responsive patients
IVIG: intravenous immunoglobulin, CRP: C-reactive protein, CAL: coronary artery lesion
Fig. 1.
The prevalence of fever in the acute phase of Kawasaki disease after intravenous immunoglobulin (IVIG) therapy without aspirin in 43 IVIG-responsive patients.
Proportion of patients with high C-reactive protein after treatment and time to decrease in C-reactive protein
The number of cases in which CRP remained high (>3 mg/dL) at 48 to 72 hours after IVIG infusion was 4 (9.3%) of the 43 patients in the study group and 10 (9.3%) of the 107 patients in the control group (p=0.242). Regarding the time to CRP <3 mg/dL, no difference was found between the study and control group (3.5±1.1 days vs. 3.6±1.2 days, p=0.839) (Table 4).
Incidence of coronary artery lesions
There was no significant difference in the incidence of CALs between the study and control group {2/43 (4.7%) vs. 3/107 (2.8%), p=0.625} (Table 4).
Comparisons between the study and the control group in the intravenous immunoglobulin-resistant patients
Between the study and control group, there were also no differences in the time to decrease in CRP (6.5±2.6 days vs. 6.3±3.8 days, p=0.889) and the incidence of CALs {0/8 (0%) vs. 7/22 (31.8%), p=0.143}.
Discussion
Aspirin has been a part of standard therapy because of its anti-inflammatory, antipyretic, and antiplatelet effects since it was used in the treatment of acute KD prior to IVIG era. With regard to hyper-coagulable state in KD,14) moderate-dose aspirin (30 mg/kg/day) may be advantageous over high-dose aspirin (100 to 150 mg/kg/day) because of more suppression of platelet aggregation15) and less inhibition of prostacyclin production.16) However, high-dose aspirin has been shown to inhibit shear-induced platelet reaction involving thrombin generation.17)
Focusing on the prevention of CALs, there have been attempts to determine the optimal dose of aspirin in acute KD. In patients treated with only aspirin without IVIG, no difference was found in the incidence of CALs between patients with high-dose aspirin (100 mg/kg/day) and those with moderate-dose aspirin (30 mg/kg/day).16) Two large meta analyses9),10) have shown that there is also no difference in the incidence of CALs between patients treated with high- (80 to 120 mg/kg/day) vs. moderate- (30 to 50 mg/kg/day) dose aspirin with IVIG. Recently, Hsieh et al.18) reported that treatment with only IVIG without high- or moderate-dose aspirin has no effect on clinical outcome or development of CALs. All these studies suggest that low- or moderate-dose aspirin has efficacy similar to that of high-dose aspirin in the acute phase of KD, and furthermore that there is no benefit to high-dose aspirin in the acute phase of the illness.
In this study, the overall resistance to initial IVIG was 15.7% in the study group, not statistically different from 17.1% in the control group. This incidence is compatible with those in the previous studies, in which approximately 10% to 20% of patients have persistent or recrudescent fever after initial IVIG plus aspirin therapy.19),20) This result suggests that treatment without high-dose aspirin in the acute phase may not increase unresponsiveness to IVIG therapy.
With respect to post-IVIG duration of fever according to aspirin dose, Saulsbury21) demonstrated that there was no difference between patients treated with IVIG and either high- or low-dose aspirin. In our study, 83.7% of the IVIG-responsive patients became afebrile within 24 hours after IVIG therapy without use of aspirin. However, mean duration of fever after treatment was longer in the study group compared with the control group. This result is consistent with the findings of Akagi et al.16) who showed that patients receiving high-dose aspirin had a shorter duration of fever.
We arbitrarily defined CRP <3 mg/dL as an acceptable level for resolution of inflammation by IVIG therapy, which was derived from the criterion of the American Heart Association for the evaluation of suspected incomplete KD.22) We found no difference between patients treated with versus without aspirin in the proportion of patients who maintained a high CRP at 48 to 72 hours after treatment and also in mean time to CRP <3 mg/dL. These findings suggest that treatment without aspirin may not affect the resolution of inflammation achieved by IVIG infusion.
The primary goal of treating KD patients is to prevent CALs. With regard to the incidence of CALs, we found that treatment without aspirin may not increase the development of CALs, and this finding is in an agreement with the study of Hsieh et al.18) Therefore, we need to reassess the efficacy of high-dose aspirin in the acute treatment of KD. It seems unwise to expose children to high-dose aspirin therapy when the available data show no appreciable benefit in preventing CALs.
The limitation to this study is the small number of patients, which may weaken statistical analytic power. In addition, the study on control group was performed at different times. As CRPs were not measured daily, error may exist in the time to decrease in CRP. Finally, the use of Japanese Ministry of Health and Welfare criteria may underestimate the true incidence of CALs in KD patients.23)
In conclusion, the treatment without high-dose aspirin in the acute phase of KD has no influence on the response to IVIG, decline in inflammation, or the development of CALs. Therefore, high-dose aspirin may provide little benefit to the treatment of acute KD. Future prospective study with a larger number of patients, ideally a randomized trial, is needed to establish the true efficacy of high-dose aspirin for the acute therapy of KD in the IVIG era.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Dajani AS, Taubert KA, Gerber MA, et al. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.cir.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 2.Kusakawa S, Tatara K. Efficacies and risks of aspirin in the treatment of the Kawasaki disease. Prog Clin Biol Res. 1987;250:401–413. [PubMed] [Google Scholar]
- 3.Matsubara T, Mason W, Kashani IA, Kligerman M, Burns JC. Gastrointestinal hemorrhage complicating aspirin therapy in acute Kawasaki disease. J Pediatr. 1996;128(5 Pt 1):701–703. doi: 10.1016/s0022-3476(96)80140-5. [DOI] [PubMed] [Google Scholar]
- 4.Sundel RP, Newburger JW, McGill T, et al. Sensorineural hearing loss associated with Kawasaki disease. J Pediatr. 1990;117:371–377. doi: 10.1016/s0022-3476(05)81075-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Hung HY, Huang FY. Kawasaki disease with Reye syndrome: report of one case. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1992;33:67–71. [PubMed] [Google Scholar]
- 6.Koren G, MacLeod SM. Difficulty in achieving therapeutic serum concentrations of salicylate in Kawasaki disease. J Pediatr. 1984;105:991–995. doi: 10.1016/s0022-3476(84)80097-9. [DOI] [PubMed] [Google Scholar]
- 7.Koren G, Schaffer F, Silverman E, et al. Determinants of low serum concentrations of salicylates in patients with Kawasaki disease. J Pediatr. 1988;112:663–667. doi: 10.1016/s0022-3476(88)80194-x. [DOI] [PubMed] [Google Scholar]
- 8.Koren G, Silverman E, Sundel R, et al. Decreased protein binding of salicylates in Kawasaki disease. J Pediatr. 1991;118:456–459. doi: 10.1016/s0022-3476(05)82168-7. [DOI] [PubMed] [Google Scholar]
- 9.Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995;96:1057–1061. [PubMed] [Google Scholar]
- 10.Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997;131:888–893. doi: 10.1016/s0022-3476(97)70038-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim HK, Oh J, Hong YM, Sohn S. Parameters to guide retreatment after initial intravenous immunoglobulin therapy in kawasaki disease. Korean Circ J. 2011;41:379–384. doi: 10.4070/kcj.2011.41.7.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 13.Research committee on Kawasaki disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. Tokyo: Ministry of Health and Welfare; 1984. [Google Scholar]
- 14.Burns JC, Glode MP, Clarke SH, Wiggins J, Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984;105:206–211. doi: 10.1016/s0022-3476(84)80114-6. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama T, Kato H, Ichinose E. Aspirin treatment and platelet function in Kawasaki disease. Kurume Med J. 1980;27:57–61. doi: 10.2739/kurumemedj.27.57. [DOI] [PubMed] [Google Scholar]
- 16.Akagi T, Kato H, Inoue O, Sato N. A study on the optimal dose of aspirin therapy in Kawasaki disease--clinical evaluation and arachidonic acid metabolism. Kurume Med J. 1990;37:203–208. doi: 10.2739/kurumemedj.37.203. [DOI] [PubMed] [Google Scholar]
- 17.Ratnatunga CP, Edmondson SF, Rees GM, Kovacs IB. High-dose aspirin inhibits shear-induced platelet reaction involving thrombin generation. Circulation. 1992;85:1077–1082. doi: 10.1161/01.cir.85.3.1077. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh KS, Weng KP, Lin CC, Huang TC, Lee CL, Huang SM. Treatment of acute Kawasaki disease: aspirin's role in the febrile stage revisited. Pediatrics. 2004;114:e689–e693. doi: 10.1542/peds.2004-1037. [DOI] [PubMed] [Google Scholar]
- 19.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP US/Canadian Kawasaki Syndrome Study Group. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 21.Saulsbury FT. Comparison of high-dose and low-dose aspirin plus intravenous immunoglobulin in the treatment of Kawasaki syndrome. Clin Pediatr (Phila) 2002;41:597–601. doi: 10.1177/000992280204100807. [DOI] [PubMed] [Google Scholar]
- 22.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 23.de Zorzi A, Colan SD, Gauvreau K, Baker AL, Sundel RP, Newburger JW. Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr. 1998;133:254–258. doi: 10.1016/s0022-3476(98)70229-x. [DOI] [PubMed] [Google Scholar]