Table 1. Parameters employed.
| Parameter | Interpretation | Value |
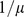
|
Average duration of the maternal antibodies protection | 6 months [23] |
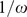
|
Average duration of the latency period | 14 days [27] |
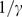
|
Average duration of the varicella infectivity period | 7 days [27] |
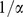
|
Average duration of the HZ infectivity period | 7 days [27] |
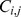
|
Age specific contact matrices (different for each countries) | based on census data [31] |
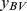
|
Relative VZV infectiousness of breakthrough varicella cases | 0.5 [43] |
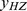
|
Relative VZV infectiousness of HZ cases | 0.05 [23] |
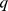
|
Adjusting factor for contacts relevant for VZV transmission | fitted against the VZV seroprevalence |

|
Adjusting factor for contacts relevant for the boosting of CMI (boosting component) accounting for the uncertainty of FOI in adults and the elderly | fitted against HZ incidence |
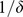
|
Average duration of the CMI | fitted against HZ incidence |
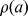
|
Age dependent VZV reactivation rate 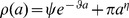
|
fitted against HZ incidence |
| T | Vaccine take (probability) | unif. sampled from (0.8,1) [15], [17], [37], [39], [42] |
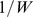
|
Average duration of vaccine waning immunity | unif. sampled from (1,200) years [52] |
|
Reduction factor of the risk of developing HZ for vaccinees | unif. sampled from (1/12,1/4) [44] |
Model parameters, description and values considered for simulations.
