Figure 2.
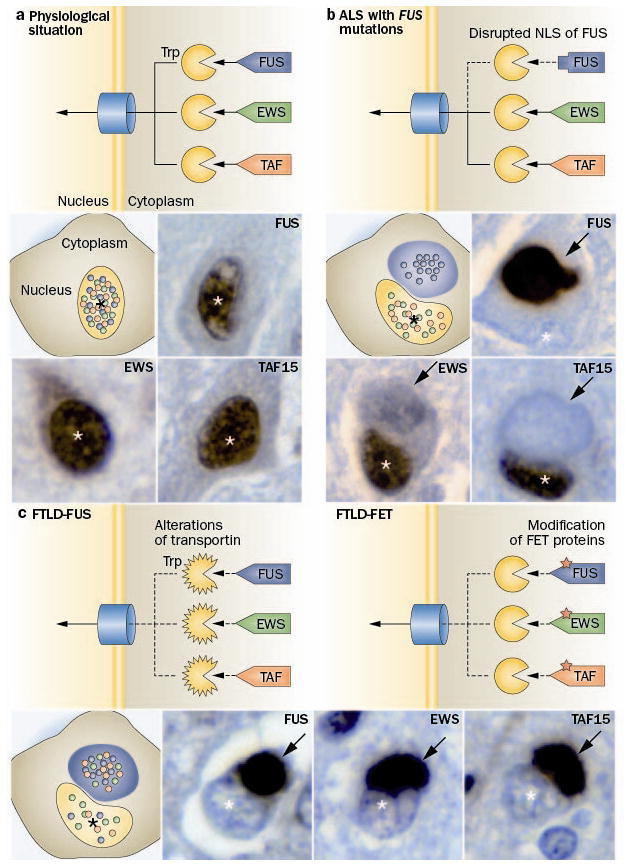
Distinct pathomechanisms of ALS-FUS and FTLD-FUS. a The FET protein members, FUS, EWS and TAF15 contain a proline-tyrosine nuclear localization signal (PY-NLS, triangle) which is bound by the receptor protein Transportin (Trp). This mediates the transport of these proteins across the nuclear pore complex into the nucleus, resulting in a predominant nuclear localization of all three proteins under physiological conditions. b In ALS with FUS mutations, the PY-NLS of FUS is disrupted due to mutations (rectangle) leading to an impaired interaction with Trp and nuclear import of FUS, while TAF15 and EWS are normally transported to the nucleus. This results in a selective accumulation of FUS into cytoplasmic inclusion in ALS-FUS patients, with retained nuclear localization of TAF15 and EWS. c In contrast, FTLD-FUS patients show co-accumulation of all FET proteins into cytoplasmic inclusions accompanied with their nuclear depletion. This complex dysregulation of all FET proteins in FTLD-FUS might be explained by either of two broad scenarios: c-1 alterations of Trp itself, e. g. by genetic variations, reduced expression levels or posttranslational modifications; or c-2 posttranslational modifications of FET proteins, which interfere with proper Trp binding. Each photomicrograph shows a single neuron with a cytoplasmic inclusion (arrow) and the nucleus indicated by the asterisk (*), immunostained for the FET protein indicated (brown stain).
