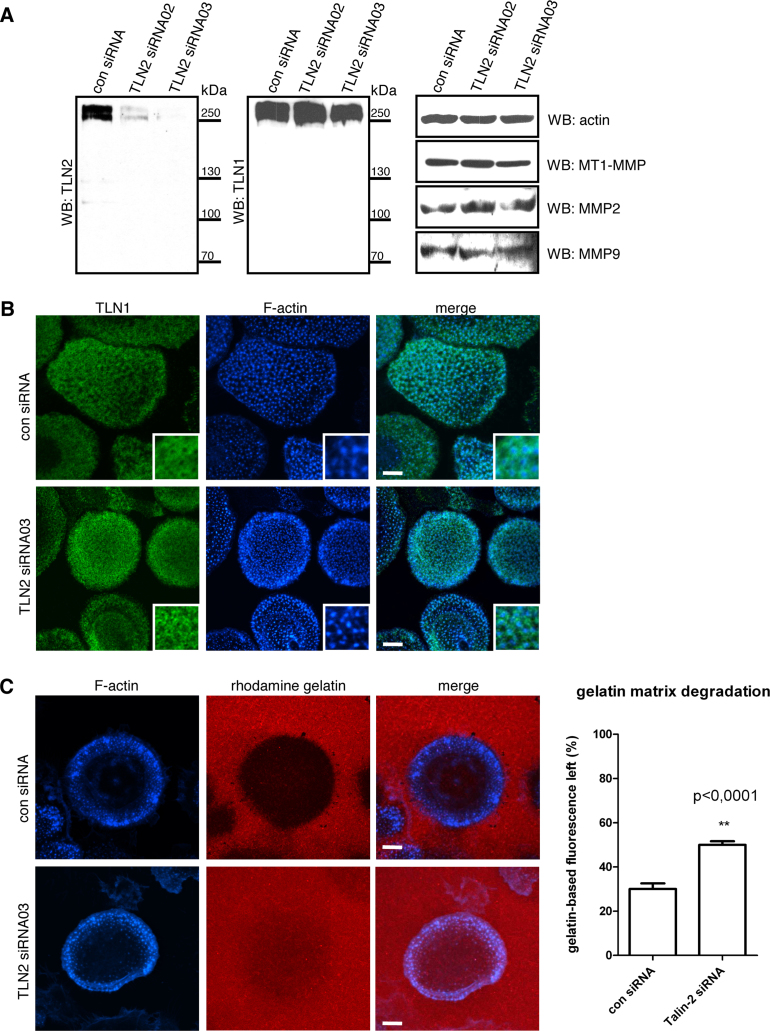
Fig. 4.
Knock-down of talin2 leads to a reduction of podosomal matrix degradation in primary human macrophages. (A) Western blots of macrophage lysates 72 h after transfection with the talin2 siRNAs indicated. Blots were probed for; left – talin2 (Mab 121A1), middle – talin1 (Mab 97H6), right – β-actin loading control and the matrix metalloproteinases (MMP) indicated. MMP2 and MMP9 rabbit polyclonal antibodies were obtained from Santa Cruz and the anti MT1-MMP antibody was from Millipore. (B) Primary human macrophages transfected with a control luciferase siRNA (upper panels) or a talin2 siRNA (lower panels) for 72 h, were seeded on coverslips, stained for talin1 (green) or F-actin (blue) and imaged by confocal laser scanning microscopy. Note localisation of talin1 to podosome rings surrounding F-actin-rich cores. (C) Knockdown of talin2 reduces podosome-dependent gelatin matrix degradation. Human macrophages transfected with a talin2 siRNA or control luciferase siRNA for 72 h were seeded on a rhodamine-labelled gelatin matrix for 6 h, and then fixed and stained for F-actin (blue). Dark areas indicate the region of matrix degradation. The degree of gelatin matrix degradation was quantified (±SD) by measuring the primary fluorescence under each of 20 cells using confocal laser scanning microscopy. The experiments were conducted in triplicate. The fluorescence intensity of the undegraded labelled matrix was set at 100%. Asterisks indicate a P value < 0.0001. Magnification bar = 10 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)