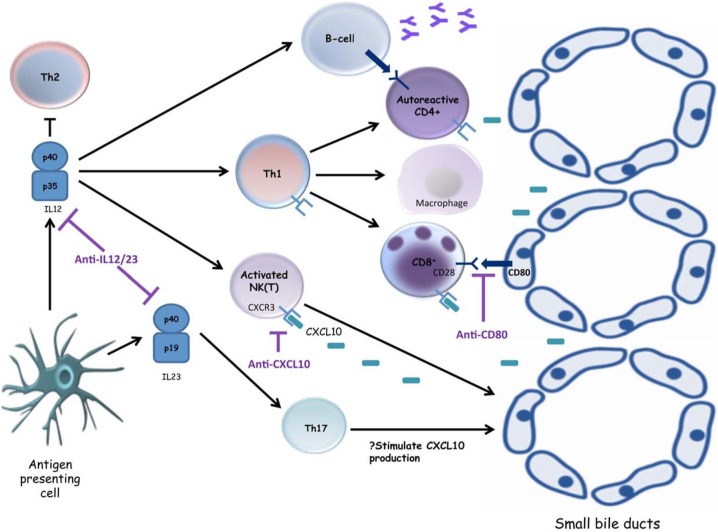
Figure 3.
Emerging small-molecule inhibitors in primary biliary cirrhosis. Interleukin (IL)-12 secretion by an activated antigen presenting cell leads to natural killer (NK) cytotoxicity, macrophage activation and T helper 1 (Th1) differentiation, with subsequent activation of cytotoxic CD8+ T cells as well as antibody-secreting B cells, all of which potentiate the destruction of cholangiocytes. The p40 subunit of IL12 is also a component of IL23, an essential cytokine for the differentiation of Th17 cells. Targeting the p40 subunit has been shown to ameliorate experimental immune-mediated cholangiopathy, and it is hoped these promising results can be translated into clinical practice. The immune-mediated destruction of small-sized bile ducts in primary biliary cirrhosis (PBC) is predominantly cell mediated, characterized by Th1 cells, CD8+ T cells, NK cells and NKT cells which express CXCR3. Neutralizing antibodies to CXCL10, a ligand for CXCR3, may offer the possibility to interfere with one of the key inflammatory processes contributing to immune-mediated biliary destruction in PBC. Blockade of costimulatory signals between T cells expressing CD28 and antigen-presenting cells expressing CD80 (e.g. cholangiocytes, antibody-secreting B cells) could also represent an important approach for the treatment of autoimmune diseases.