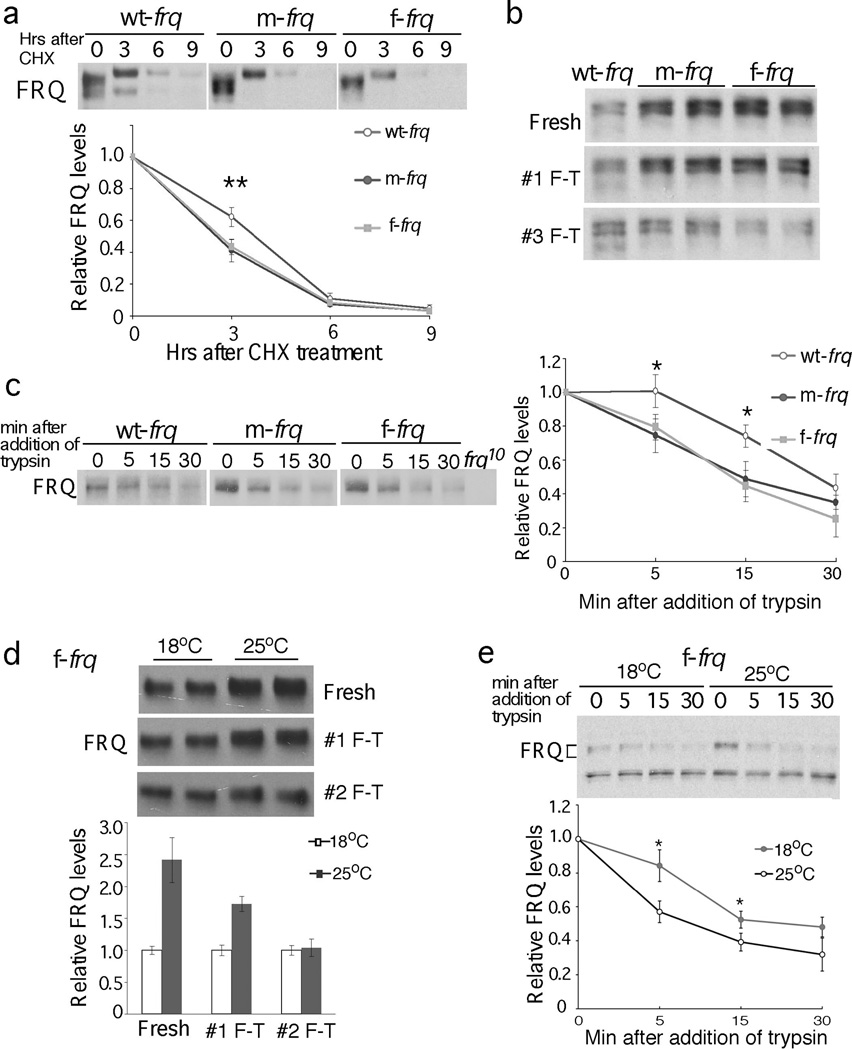
Figure 3.
FRQ protein in the codon-optimized strains is less stable and more sensitive to trypsin digestion. (a) Western blots showing FRQ degradation after CHX treatment (10 µg/ml). A longer exposure for the wt-frq strain was used so that the FRQ signals at time 0 are comparable in three strains. Densitometric analyses of results of four independent experiments are shown. Error bars, standard deviation. (b & c) Western blots showing sensitivity of FRQ from codon-optimized strains to freeze-thaw cycles (b) and trypsin (1 µg/ml) digestion (c). A longer exposure for the wt-frq strain was used in (c). Densitometric analyses of FRQ levels of three independent experiments in are shown. (d & e) Western blots showing that FRQ from the f-frq strain grown at 18°C is more resistant to freeze and thaw cycles (d, n=2) and to trypsin digestion (e, n=4) than that from 25°C. Two asterisks indicate p value <0.01, and one asterisk indicates p value <0.05.