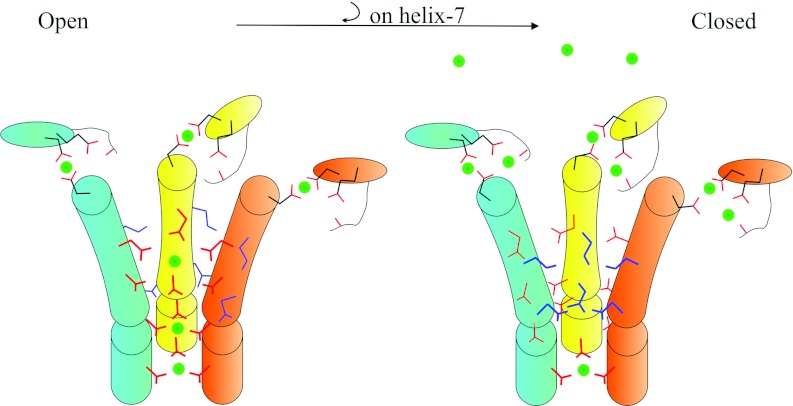
Figure 8. Illustrative model of the mechanisms of Co2+ transport and gating by TmCorA.
The cartoons represent helix 7 (cylinders) and the α-β regions (ovals) from three (out of five) monomers (cyan, yellow and orange). When the channel is open, the hydroxy-group-containing side chains of Thr305, Thr299, Thr295 and Asn288 (red lines) face the channel (thicker lines) and co-ordinate the transport of partially hydrated Co2+ (green circles). The M1 site is also occupied by Co2+ through tight interactions with Asn89 (on the α-β region) and Asp253 (on helix 7). Co2+, once reaching high concentrations within the cytoplasm, occupies the M2 site. This binding pulls Asp253 and causes a clockwise rotation of helix 7 along its axis. This rotation will then remove the polar residues from the channel and replace them with the hydrophobic residues (blue lines), which prevents ion movement through the channel.