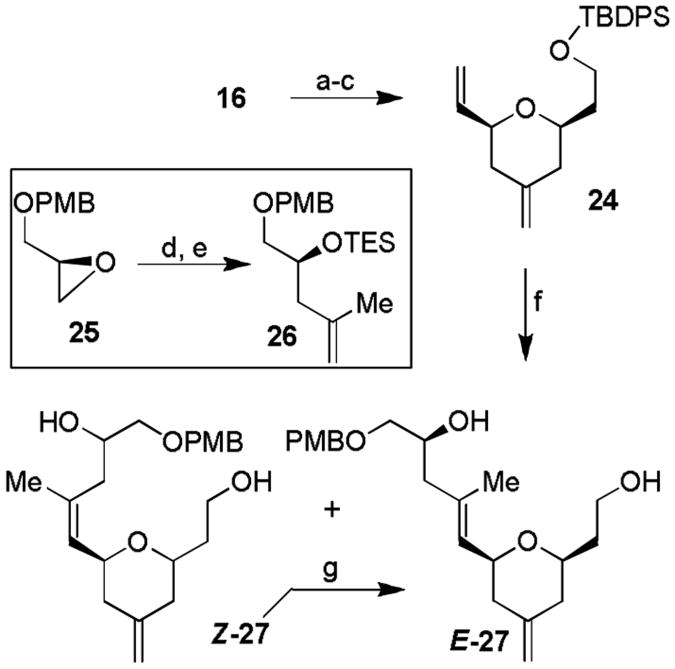
Scheme 3.
Synthesis of tetrahydropyran E-15. Reagents and conditions: a) AD-mix-α (5 mol-% based on osmium), MeSO2NH2 (1.5 equiv.), t-BuOH/H2O (1:1), 23 °C, 1 h; b) NaIO4 (1.2 equiv.), MeOH/H2O, 23 °C, 2 h; c) Ph3P=CH2 (4.5 equiv.), THF, 40 °C, 16 h. 78% overall yield three steps; d) isopropenylMgBr, (2 equiv.), Li2CuCl4 (5 mol-%), THF, 0 °C, 2 h, 91%; (e) TESOTf (1 equiv.), 2,6-lutidine (1.5 equiv.), CH2Cl2, −30 °C, 1 h, 94%; f) Grubbs II (10 mol-%), 26 (10 equiv.), CH2Cl2, reflux, 9 h, 57%; g) HF/Py, THF, 23 °C, 14 h, E/Z = 1.7:1, overall 81%; h) hv, benzene, 23 °C, 4 h, 51% after two runs.