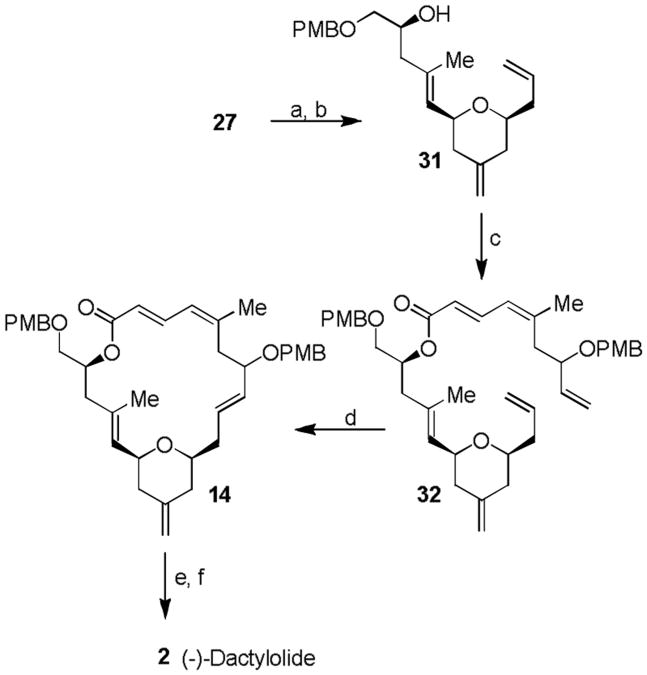
Scheme 5.
The synthesis of (-)-dactylolide 2. Reagents and conditions: a) PhI(OAc)2 (2.3 equiv.), TEMPO (0.8 equiv.), CH2Cl2, 23 °C, 8.5 h; b) PPh3CH2 (3.5 equiv.), THF, 40 °C, 12 h, 60% in two steps; c) 2,4,6-trichlorobenzoyl chloride (1.5 equiv.), Et3N (2.6 equiv.), DMAP (1 equiv.), toluene, 23 °C, 20 h, 91%; d) Grubbs II (12 mol-%), benzene, 60 °C, 20 h; e) DDQ (2.2 equiv.), CH2Cl2/water, 23 °C, 30 min, 65% in two steps; f) Dess-Martin reagent (6.0 equiv.), NaHCO3 (13.8 equiv.), CH2Cl2, 23 °C, 3.5 h, 80%. DMAP = N, N-dimethyl-4-aminopyridine. TEMPO = 2,2,6,6-Tetramethylpiperidine 1-oxyl.