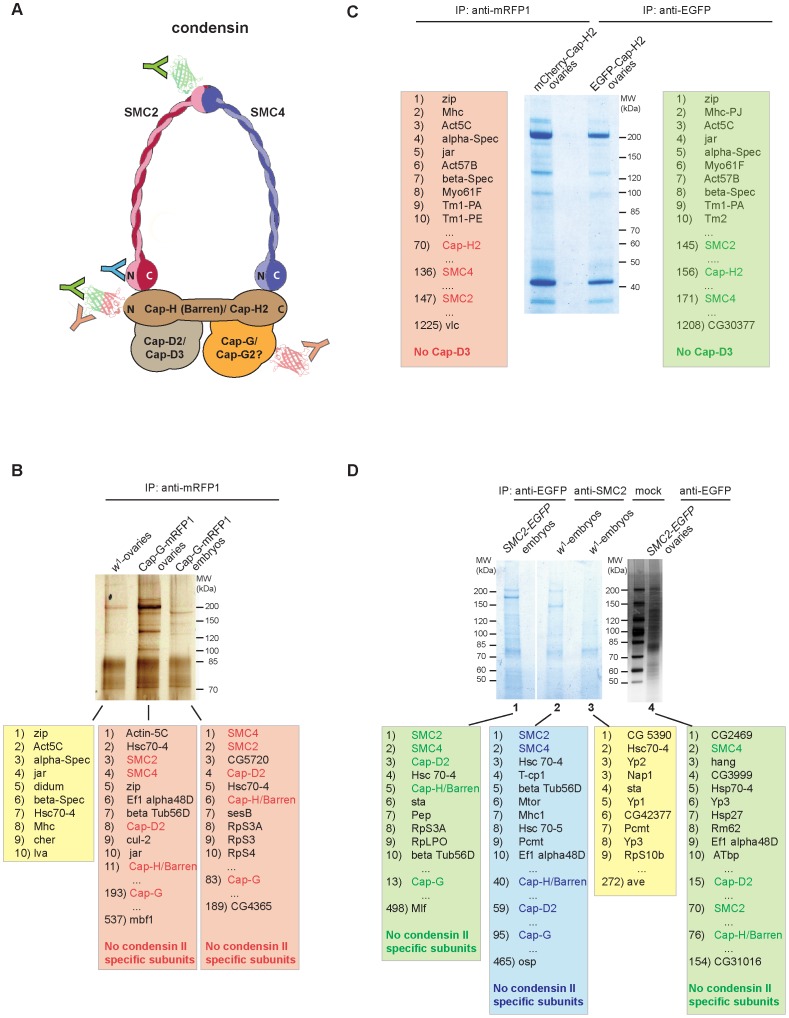
Figure 4. Cap-G is not associated with condensin II–specific subunits in vivo.
(A) Schematic model of a condensin complex with the positions indicated that are recognized by antibodies used in the immunoprecipitation experiments. Fused EGFP or mCherry/mRFP tags are depicted as green or red barrel-like structures, respectively. The red/green colored barrel fused to the N-terminus of Cap-H2 indicates that fusions with both EGFP and mCherry were analyzed as shown in panel (C). Color coding of the antibodies (Y) corresponds to the color shading of the boxes with the lists of identified proteins within the precipitates shown in panels (B–D). (B) Protein extracts from 3–6 h old embryos expressing gmRFP1-Cap-G as well as from ovaries of wild type females (w1) or females expressing gmRFP1-Cap-G were subjected to immunoprecipitation with anti-mRFP antibodies. Precipitated proteins were separated by SDS-PAGE and visualized by silver staining and in a parallel experiment with colloidal Coomassie Blue. Coomassie Blue stained lanes were processed for mass spectrometric (MS) analysis. (C) Ovary extracts derived from females expressing UASP1-mCherry-Cap-H2 or UASP1-EGFP-Cap-H2 driven by tubP-Gal4 were subjected to immunoprecipitation with anti-mRFP1 antibodies. Immunoprecipitates were separated by SDS-PAGE, stained with colloidal Coomassie Blue and processed for MS analysis. (D) Protein extracts from 3–6 h old embryos expressing gSMC2h-EGFP (lane 1) or wild type embryos (lanes 2 and 3) as well as from ovaries of females expressing gSMC2h-EGFP (lane 4) were subjected to immunoprecipitation using anti-EGFP antibodies (lanes 1 and 4), anti-SMC2-antibodies (lane2) or were mock treated with beads only (lane 3). Immunoprecipitates were separated by SDS-PAGE, stained with colloidal Coomassie Blue (lanes 1–3) or a silver stain (lane 4) and processed for MS analysis directly (lanes 1–3) or after a parallel SDS-PAGE subsequently stained with colloidal Coomassie (lane 4). In each case, the list of identified Drosophila proteins was sorted according to the cumulative peptide intensities. The top ten ranked proteins, condensin subunits, and the lowest ranked entries are listed.