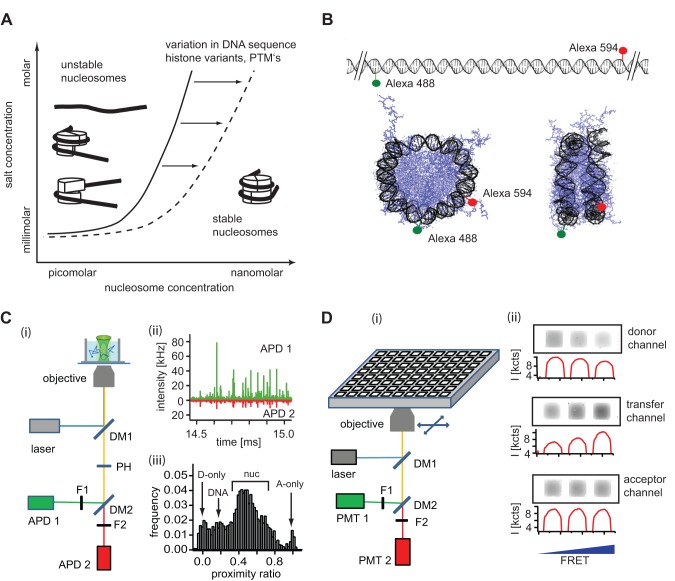
Figure 1. A combined single molecule – bulk FRET approach to study nucleosome stability.
A) Theoretical diagram of nucleosome stability as a function of salt and nucleosome concentration (adapted from ref. [22]). The solid line represents the amount of salt needed to destabilize nucleosomes at a given nucleosome concentration. Nucleosomes generally remain stable at higher concentrations and lower ionic strength, dissociation occurs at elevated ionic strength and nucleosome concentrations in the sub-nM range. The dashed line represents changes in nucleosome stability from altered nucleosome composition. B) DNA labeling for nucleosome FRET experiments. 170 bp long DNA fragments were labeled at positions -42 and +52 from the dyad axis. In the intact nucleosome both dyes are located ≈ 6 nm apart, allowing for FRET, while in a fully dissociated nucleosome or free DNA fragment both dyes are too far apart to undergo FRET. C) (i) Schematic of confocal single molecule detection of nucleosomes in solution. A detailed description of the setup is given in Section S1 in File S1. (ii) The passage of individual nucleosomes through the focus generates bursts of fluorescence. (iii) For each burst a proximity ratio is calculated and data binned for histogram analysis. The position of relevant subpopulations in the histogram is indicated. D) (i) Schematic setup for microplate-scanning FRET (μpsFRET). Samples are loaded into a 384-well multiplate and imaged in three spectral channels using a commercial Typhoon™ multimode scanner with confocal optics (i). Grey scale images and intensity profiles of samples with different bulk FRET efficiencies (ii). Higher FRET leads to a decrease of signal in the donor channel and a corresponding increase of signal in the transfer channel. The signal in the acceptor channel remains unaffected. From these intensities P-values are calculated for each well. Abbreviations: DM: dichroic mirror, F: emission filter, APD: avalanche photodiode, PMT, photomultiplier tube, PH: pinhole.