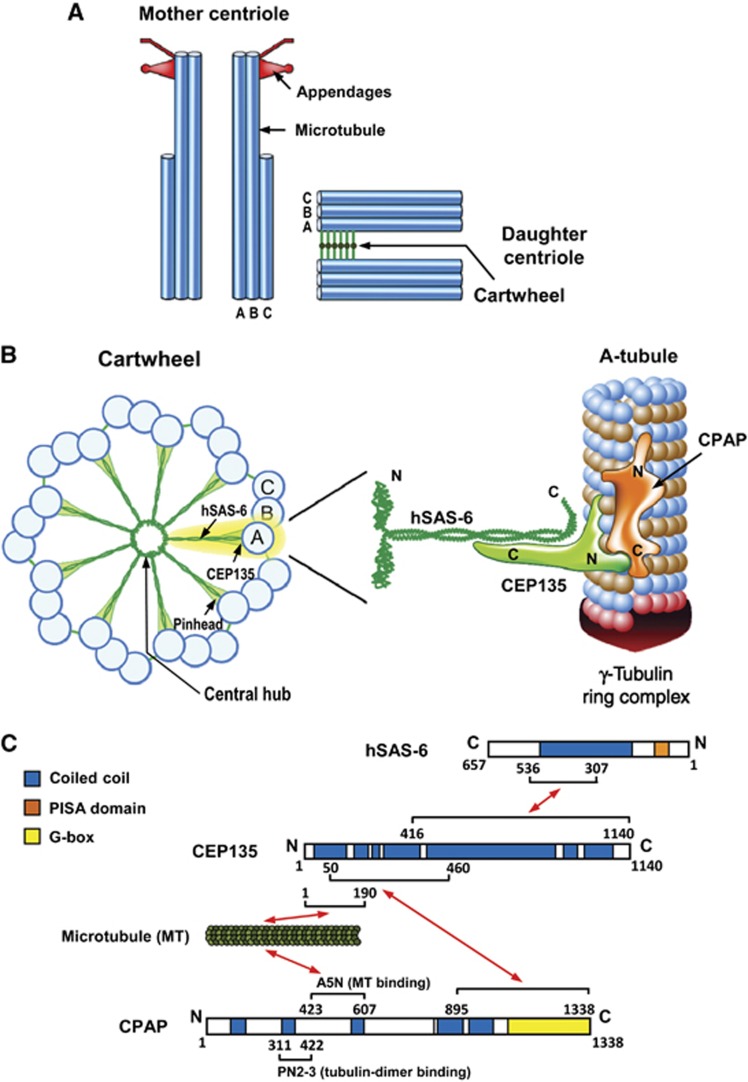
Figure 9.
A proposed model for how CEP135 stabilizes the cartwheel structure and is required for CPAP-mediated centriole elongation. (A) Schematic representation of mother and daughter centriole pair in a human cell. (B) Schematic representation of the cartwheel and its associated proteins viewed from the proximal end (left). A close-up view of the A-tubule and its associated proteins (right) is enlarged and rotated 90° from the view on the left (A-tubule). CEP135 in the pinhead serves as a bridging protein that directly interacts with hSAS-6 in the central hub via its C-terminal region, and with the outer microtubules (possibly the A-tubule) and CPAP through its N terminus. CPAP carries both a tubulin dimer-binding domain (PN2-3) (Hung et al, 2004) and a microtubule-binding domain (A5N) (Hsu et al, 2008), and is associated with the γ-tubulin complex (Hung et al, 2000). The CPAP C-terminus directly interacts with the CEP135 N-terminus and promotes centriolar microtubule assembly. The A-tubule, which contains 13 protofilaments and is end-capped by the γ-tubulin ring complex (Guichard et al, 2010), may be initiated by CPAP-trigged γ-tubulin-dependent nucleation occurring via a yet-unknown mechanism. (C) Schematic summary of the established interactions of hSAS-6, CEP135, microtubules, and CPAP, and their corresponding structural and functional domains.