Abstract
Objective
A preliminary study was conducted to investigate feasibility of using an oral cancer chemopreventive agent (−)-epigallocatechin-3-gallate (EGCG), the most biologically active component in the green tea extract, in a form of ‘swish-and-spit’ mouthwash. Such application of EGCG is beneficial as it maximizes exposure of the oral mucosa to the agent but minimizes systemic side effect.
Study design
The study was conducted on individuals suspected to have oral field cancerization who are at a high risk for developing recurrent oral precancerous and carcinomatous lesions. EGCG was used as a daily mouthwash for 7 days. EGCG’s ability to modulate target molecules implicated in oral carcinogenesis was assessed by measuring the change in expression level of biomarkers.
Results
Immunohistochemical expression of phosphoactivated epidermal growth factor receptor (pEGFR), cyclooxygenase-2 (cox-2) and ki-67 were evaluated at baseline and at the endpoint (day 8). Although not statistically significant, overall decrease in expression levels of pEGFR (27.5%), cox-2 (15.9%) and ki-67 positive cells (51.8%) were observed following EGCG treatment. Moreover, a detectable level of EGCG was found in saliva but not in plasma after the one-week treatment regime, demonstrating local availability of EGCG in oral mucosa without significant systemic absorption.
Conclusion
To best of our knowledge this is the first study to explore use of oral cancer chemopreventive agent in a form of mouthwash in patients with oral field cancerization. Although a definitive conclusion was not reached due to limited sample size, if proven effective, EGCG therapy may offer a non-invasive preventive modality for oral field cancerization.
Keywords: (−)-epigallocatechin-3-gallate, oral field cancerization, topical chemopreventive agent, feasibility trial
INTRODUCTION
Annually, an estimated 400,000 people worldwide are newly diagnosed with oral cancer, which accounts for 5% of all cancers in men and 2% in women1-3. Oral squamous cell carcinoma (OSCC) is a deadly disease with only a 50% five-year survival rate1-3. OSCCs are treated primarily by surgery with or without adjuvant radiotherapy and/or chemotherapy. However, there is significant post-treatment morbidity and mortality secondary to recurrences1-3.
OSCC recurrences may be due to the cancerized field remaining following surgery4-10. Oral cancerized field consists of atypical cells with molecular alterations but those that have not yet acquired histomorphologic change. Hence, oral field cancerization is an earliest form of precancer and the atypical epithelial cells within the field undergo malignant transformation to become invasive OSCC. The clinical implication of this concept is that after the removal of microscopically evident dysplasias and OSCC, the cancerized field may still remain in the patient resulting in recurrences. Molecular alterations have been demonstrated in these clinically and histologically normal cells within field cancerization10. The recurrent tumors arising from the same cancerized field as that of the primary carcinoma are termed ‘second field tumors (SFT)4-6.
Because genetically altered epithelial cells within the oral cancerized field do not yet exhibit morphologic change detectable by microscopic or clinical assessment, there is no standard medical modality for treatment. Chemopreventive therapy is an appealing alternative to simple observation, provided that the agent has therapeutic targets. Because oral mucosa is readily accessible, a topical chemopreventive agent can be used to maximize the local exposure and minimize systemic side effects. (−)-Epigallocatechin-3-gallate (EGCG) is the most biologically active catechin found in green tea extract Polyphenone 70A (P-70A) and exhibits anti-tumoral effects11-19. EGCG inhibited growth of a head and neck squamous cell carcinoma cell line and induced apoptosis11. It also favorably alters expression levels of phosphoactivated epidermal growth factor receptor (pEGFR), cyclooxygenase-2 (cox-2), activated Stat 3 (pStat3) and cyclin D111-15. Other studies have shown its efficacy as a topical agent when used as an intraoral cream and green tea beverage18,19.
In this pilot trial, we investigated the feasibility of utilizing EGCG mouthwash as an intervention therapy and assessing its efficacy by measuring expression levels of EGCG’s molecular targets in patients with evidence of field cancerization. Considering that the dose of EGCG tested in this study is equivalent to 8-16 cups of green tea, the amount consumed daily by heavy tea drinkers, EGCG in a form of mouthwash may prove to be a simple and potentially promising long-term treatment modality, if demonstrated to be effective. To best of knowledge, this is the first study to explore feasibility of topical application of EGCG in a form of mouthwash and assessment of its modulatory effect on the expression levels of the molecular targets in oral cancerized field.
MATERIALS AND METHODS
Study drug
Polyphenone 70A (P-70A) is a generous gift from Yukihiko Hara in the Mitsui Norin Co., Ltd., Tokyo, Japan (Lot No. 0608091). P-70A is a brown powder similar to green tea composed of 55.9% epigallocatechin gallate (EGCG) and other catechins, 2.2% epigallocatechin (EGC), 1.4% epicatechin (EC), 12.6% epicatechin gallate (ECG), 4.9% gallocatechin gallate (GCG), 0.7% catechins gallate (CG) and 0.5% gallocatechin (GC). EGCG is the most biologically active catechin in P-70A. When compared, pure EGCG and polyphenol compound (i.e. P-70A) containing same amount of EGCG demonstrated similar safety and pharmacokinetic properties as well as antitumoral potencies12,20. A dose level of 800 mg EGCG can be obtained from approximately 1.4 g of P-70A. Immediately before each use, the participants were instructed to mix 1.4 g of P-70A powder with 15 ml (3 teaspoon) of Ora-Blend (Paddock Laboratories, Inc., Minneapolis, MN) to formulate EGCG mouthwash. The IND to use P-70A solution as a mouthwash formula was obtained from the Food & Drug Administration.
Participants
Seven participants with evidence of oral field cancerization but no evidence of active OSCC were enrolled in this open-labeled, non-randomized study. Individuals with a history of one or more locally recurrent histology-confirmed oral dysplasias and/or carcinomas were considered to have oral field cancerization. These individuals are at a high risk for developing recurrences and were under close clinical observation. The participants were all more than 18 years old and were in performance status 0-1 (determined by Eastern Cooperative Oncology Group performance status). Individuals were excluded from the study if they had chemo or radiation therapy within one month (both chemotherapy and radiation-induced mucositis will resolve 2 to 3 weeks after treatment), had abnormal liver and/or renal function, untreated metabolic disorders and/or other serious acute or chronic diseases, routinely use other introaoral topical agents (cream, lozenge, etc.) at bedtime, had regular consumption of tea, participated in another chemoprevention or clinical intervention trials either within the past 3 month or concurrently, or pregnant or nursing. The protocol was approved by the Columbia University Internal Review Board and written informed consent was obtained from all participants.
Study design
Individuals with multiple oral precancerous lesions and carcinomas were identified through a Columbia University (CU) Pathology Department data base search and enrolled from the CU College of Dental Medicine, Division of Oral & Maxillofacial Surgery and CU Medical Center, Department of Head & Neck Surgery clinics. At the baseline visit, the medical history forms were completed and height, weight, blood pressure and temperature were measured. An oral examination was conducted to ensure there was no evidence of active OSCC. A blood sample was collected for a complete blood count (with differential leukocyte count) and blood chemistry analyses including hepatic function. Aliquots of plasma and unstimulated saliva samples were also obtained for baseline measurement of EGCG concentration by high performance liquid chromatography (HPLC). Oral cell samples were collected using a mouthwash technique for the analysis of biomarker expression levels by immunohistochemistry (IHC). Participants were instructed to hold the P-70A mouthwash (800 mg EGCG) in their mouth for 2 minutes and then expectorate, once a day before bedtime for a period of 7 days. Participants recorded daily administration of EGCG on a diary form, listing any side effects experienced during this period. The participants returned on the eighth day (~9 to 18 hours after last dose of medication) for an endpoint evaluation. Similar to the baseline visit, blood, saliva, exfoliative oral epithelial cell samples were collected. All study participants received a 1-week follow-up phone call for any potential adverse events related to the study.
Collection of oral epithelial cell samples and analysis for biomarker expression
At the baseline and endpoint visits, the exfoliative oral epithelial cells were collected by rinsing the mouth with 25 ml of a commercial mouthwash solution as previously described21. The sample was then transferred into a 5 ml tube within 4 hr of collection and centrifuged (Thermo IEC) at 2700 rpm for 10 min. The pellet was then transferred to a 1.7 ml microcentrifuge tube and 95% formalin was added for fixation of the epithelial cells. After 24 hr, formalin fixed cells were embedded in paraffin.
The H&E stained slides were prepared from the paraffin-embedded oral cell blocks to identify three areas containing densely localized cells. The identified areas were marked on the corresponding cell block for TMA construction. Three tissue cores (2 mm diameter) from each sample were arrayed in the recipient paraffin block, yielding a total of 42 cores. Sections (4 μm) were generated from the TMA block for H&E slide and immunohistochemical analysis.
The expression levels of five biomarkers (pEGFR, cox-2, cyclin D1, pStat3 and ki-67) were analyzed on the TMA sections by IHC. After TMA sections (4 μm) were deparaffinized, antigen was retrieved with 1mM EDTA pH 7.5 (from a 100mM stock). Slides were washed in TBS 0.05M pH7.5 and 5% defatted powdered milk in TBS-Tween was applied as a blocking agent. For endogenous peroxidase inactivation, 3% hydrogenperoxidase and 0.1% sodium azide were applied. Slides were incubated overnight with the following previously characterized primary antibodies at the indicated dilution22-34: anti-pEGFR (Tyr 1173) (1:50; Santa Cruz Biotechnolgy); anti-cyclin D1 (1:50, 1:100, 1:200, 1:600; Santa Cruz Biotechnology); anti-pStat3 (1:50, 1:100, 1:200, 1:600; Cell Signaling); anti-cox2 (1:400; Santa Cruz Biotechnology); anti-ki67 (1:100; Santa Cruz Biotechnology). The primary antibodies were then labeled with 50 μl of HRP (Horseradish Peroxidase) conjugated secondary antibody (DakoCytomation, Envision+RSystem Labelled Polymer-HRP Anti-rabbit-HRP), washed, counterstained with hematoxylin, dehydrated and mounted. Proper control was used with each batch. For each case, the staining intensities for the biomarkers were scored from 0 (no staining) to +1 (strong staining) independently by two pathologists. In case where the interpretation was discordant, a final consensus was reached based on discussion between the two pathologists. From all cells within a TMA core, the fraction of cells staining positive were assessed and multiplied by the staining intensity score to obtain the final score as previously described35. For example, if only half of the epithelial cells in the TMA core show +1 staining, the final score would be 0.5. The final scores from three TMA cores generated from the same sample were then averaged to yield one final score per sample. The percentage of epithelial cells with nuclear staining of ki-67 was counted and recorded.
HPV in-situ hybridization (ISH)
The most recently available oral tissue biopsy samples from the participants were assessed for evidence of high-risk human papilloma virus (HPV) infection. Oncogenic virus, such as HPV high risk subtypes, have been implicated not only in oral cancer but also in a wide variety of cancers including carcinoma of the pharyngeal tonsil, larynx, esophagus, uterine cervix, vulva and penis1-3. High-risk HPV encods proteins E6 and E7 that are thought to promote degradation of p53 and pRb tumor suppressor gene products, respectively1-3. In situ hybridization was performed to detect HPV DNA. The probes for HPV low-risk that are known to hybridize with HPV genotypes 6, 11, 42, 43 and 44, and, HPV high-risk for HPV genotypes 16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68 and 70 were used. Slides were scored as positive for HPV if a punctate nuclear stain was present.
Collection of saliva and plasma for EGCG concentration measurement
Unstimulated saliva was collected by allowing the saliva to accumulate in the mouth and then expectorating into the tubes containing 20 μl of ascorbic acid-EDTA solution. A 500 μl aliquot of saliva was mixed with an equal volume of 60% acetonitrile and was stored at −80°C within 1 hour of collection. Plasma was collected in a tube containing sodium heparin, centrifuged and stored at −80°C for HPLC analysis. EGCG concentration in plasma and saliva samples was determined within 2 days of collection using a published HPLC procedure36,37. EGCG was extracted from 500 μl aliquot of saliva and 400 μl of plasma using ethanol at 4 °C and 2 hr at 300 rpm. Extracted EGCG was dissolved in 10% aqueous methanol and small aliquots (50-100 μl) were processed through reverse-phase HPLC. EGCG was eluted at a uniform flow rate of 1 ml/min throughout the HPLC run, then quantitated by UV at 280 nm using a standard curve ranging from 0.25-50 μg/ml.
Statistical analysis
The goal of this study was to assess feasibility, which was defined as the ability to measure pre and post-treatment expression levels of the biomarkers. We considered this study to be feasible if biomarkers were measurable in 5 out of 7 subjects. The lower 95% confidence limit for the feasibility rule is 40%. Safety endpoint was the primary endpoint. The secondary endpoint of the study was the completion of the 7 day once a daily 800 mg EGCG dose regime.
The differences between baseline and post-EGCG oral suspension treatment were determined with regard to the expression levels of biomarkers in the exfoliative oral epithelial cells. Because of the limited sample size, descriptive statistics was used to summarize each biomarker’s expression levels. Wilcoxon-signed ranks test (nonparametric test) was used to compare the differences in marker expression levels at baseline and the endpoint. The null hypothesis was that expression of biomarker would not change under the treatment of EGCG. A significance level of 0.05 was used to reject the null hypothesis for non-directional test.
RESULTS
Participant Characteristics
A total of 7 subjects participated in the study. The subject characteristics are summarized in Table 1. Of these, 6 were male and one was female. The average age was 64 (range 46-74). Each subject had at least one recurrent lesion involving the oral mucosa following a complete surgical removal of the initial lesion. The initial lesion and the subsequent recurrences had histologic diagnoses of epithelial dysplasia or OSCC. For example, subject 1 had epithelial dysplasia, moderate, involving the left lateral tongue diagnosed in 1999. The lesion recurred in the same location in 2002 (diagnosed as epithelial dysplasia mild-moderate) and 2003 (diagnosed as carcinoma-in-situ). After a complete removal, the lesion recurred in 2006 involving the left lateral tongue and also the anterior ventral tongue, which was diagnosed as carcinoma-in-situ. Tobacco and alcohol use are dominant risk factors for OSCC and its recurrences. They show a multiplicative effect in the oral cavity and account for 75% of the disease burden of oral malignancies1-3. In our study, six subjects never smoked and one reported smoking cessation 20 years previously. All denied history of alcohol abuse.
Table 1.
Participant Characteristics
| Subject | Year of Initial Lesion / Histologic Diagnosis |
Number of Recurrences |
Locations |
|---|---|---|---|
| 1 | 1999 / Epi. Dys. (Mod) | 3 | R Lat.& Vent. Tongue |
| 2 | 2006 / OSCC (Mod) | 1 | R & L Lat. Tongue |
| 3 | 1999 / Epi. Dys. (Mod) | 4 | L Lat. Tongue & L cheek |
| 4 | 2001 / Epi. Dys. (Mild) | 6 | R Mandibular Gingiva |
| 5 | 2006 / Epi. Atypia | 3 | R Lat Tongue & R Tonsil |
| 6 | 2000 / Epi. Dys. (Severe) | 5 | R Lat & Vent. Tongue |
| 7 | 1998 / Epi. Dys. (Mild) | 6 | R & L Lat. Tongue |
R: right; L: left; Lat: lateral; Vent: ventral; Epi: epithelium; Dys: dysplasia; Mod: moderate
Safety endpoint and participant compliance
Safety endpoint is the primary endpoint of this study. The study would be considered to be feasible if the biomarker expression levels are measureable on 5 out of 7 subjects. Therefore, if three or more patients had mild adverse events (GI upset, headache, heartburn, excess gas, nausea, dizziness, muscle pain, symptoms related to liver toxicity) or a serious adverse event (rash) such that they are placed ‘off-study’, the study would be rendered infeasible because we could not measure post marker levels on 5 or more subjects as defined above for feasibility. No one reported an adverse event that required the subject to be placed off study. At the endpoint visit, one subject reported an adverse event of mild heartburn (grade 1) during the first 2 days of EGCG use. No serious adverse events were reported. All subjects completed the study and hence the primary endpoint was reached.
The secondary endpoint was the completion of the 7 day once a daily 800 mg EGCG dose regime. All enrolled subjects presenting for analysis at the endpoint clinic visit on day 8 were to be evaluated and compliance with the daily treatment determined. Those who missed two or more daily doses of EGCG would be excluded from the analysis. All individuals in our study were compliant with the 7-day EGCG treatment regime and returned for the endpoint evaluation. We were able to collect oral cells, saliva and blood samples from all subjects at baseline and the endpoint.
EGCG concentration in saliva and plasma
There were no measurable amounts of EGCG in blood and saliva samples collected at baseline. After 7-days of daily topical treatment with 800mg EGCG, all seven subjects showed measureable local EGCG concentrations in saliva. Two of the participants noted to have dry mouth at the time of oral examination showed high levels of EGCG salivary concentration at the endpoint. None revealed evidence of detectable EGCG systemic absorption. These results are shown in Table 2.
Table 2.
Local salivary concentration versus plasma concentration of EGCG at baseline and the endpoint
| Subject | Saliva (μg/ml) | Plasma (μg/ml) | ||
|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | |
| 1 | ND | 0.58 | ND | ND |
| 2 | ND | 8.06 | ND | ND |
| 3 | ND | 0.48 | ND | ND |
| 4 | ND | 5.38 | ND | ND |
| 5 | ND | 1.77 | ND | ND |
| 6 | ND | 0.28 | ND | ND |
| 7 | ND | 0.85 | ND | ND |
ND: not detected
Standard curve range for HPLC: 0.25 – 50 μg/ml
Presence of Human Papillomavirus (HPV)
HPV high risk subtypes, especially subtypes 16, 18, 31 and 33 are thought to play a role in oral carcinogenesis3. When in situ hybridization was performed, all seven subjects were negative for both the HPV low-risk (genotypes 6, 11, 42, 43 and 44), and, HPV high-risk (genotypes 16, 18, 31, 33, 35, 45, 51, 52, 56, 58, 59, 68 and 70). (data not shown).
Effect of EGCG on the expression levels of pEGFR, cox-2, and ki-67
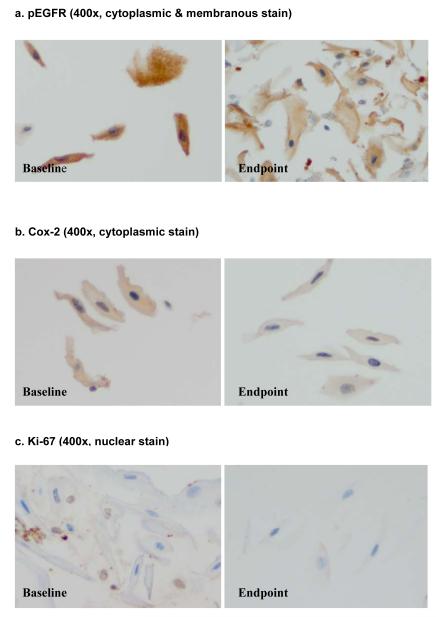
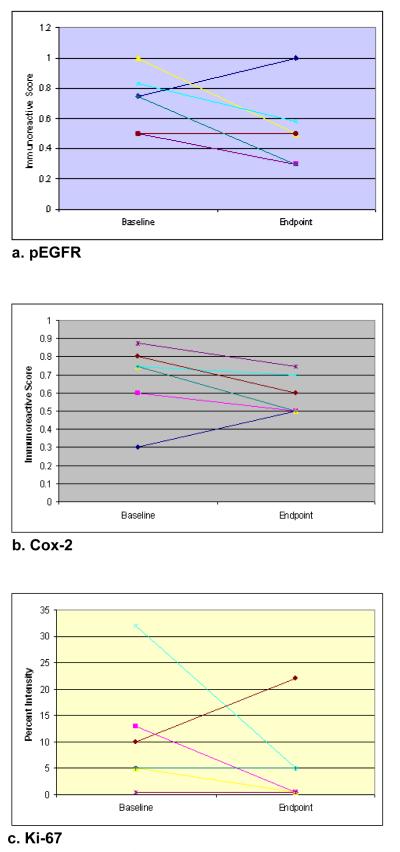
Marker expression in oral epithelial cells is shown in Figure 1, the individual trend in marker expression level in Figure 2, and the overall change in marker expression level is shown in Table 3. Each 2 mm-diameter TMA core contained approximately 60 cells, which is adequate for immunohistochemical analysis. These ~60 epithelial cells made up 40% of each TMA core and the remaining 60% consists of keratotic debris and inflammatory cells. Based on sample availability, we obtained one to three TMA cores from each cell block. Each of three TMA cores from same subject demonstrated similar staining intensity score.
Fig. 1.
Photomicrograph of marker expression in the exfoliated oral epithelial cells
Fig. 2.
Individual trends for changes in marker expression levels.
Table 3.
Changes in marker expression levels
| Markers |
Baseline Mean (SD) |
Endpoint Mean (SD) |
% Decrease |
Paired t-test | p |
|---|---|---|---|---|---|
| pEGFR | 0.69 (0.20) | 0.50 (0.25) | 27.5 | 1.98 | 0.099 |
| Cox-2 | 0.69 (0.19) | 0.58 (0.11) | 15.9 | 1.88 | 0.113 |
| Ki-67 | 10.1% (10.5) | 4.9% (7.9) | 51.8 | 1.16 | 0.292 |
For pEGFR, the cytoplasmic and membranous staining was assessed and for cox-2, the cytoplasmic staining was analyzed. The percentage of cells exhibiting positive nuclear ki-67 expression (proliferation index) was measured. We tried various dilutions for cyclin D1 and pStat3 (1:50, 1:100, 1:200, 1:600) but failed to obtain detectable stain.
pEGFR showed a decrease after EGCG treatment in 4 subjects, increased in one and stayed the same in one. Overall, the pEGFR marker expression level decreased from 0.69 (SD=0.20) at baseline to 0.50 (SD=0.25) at the endpoint, a 27.5% decrease. Cox-2 expression showed a decrease in 6 subjects. There was 15.9% reduction in cox-2 expression from baseline (0.69) to the endpoint (0.58). The percentage of epithelial cells expressing ki-67 also decreased from 10.1% at baseline to 4.9% at the endpoint. One subject showed increase in both pEGFR and Cox-2 expression levels while demonstrating decrease in proliferation index (ki-67). Since both inflammation and cigarette smoking may induce pEGFR and Cox-2 expression23, it may be that the subject suffered from traumatic injury to oral mucosa (i.e. cheek biting) or had engaged in social smoking that was not reported at the time of endpoint evaluation.
DISCUSSION
Oral cancer chemoprevention therapy offers unique opportunity to intervene malignant progression and prevent future recurrences. Oral cancerized field is the earliest form of precancerous change and may reach a considerable size (> 7 cm in diameter). Since these fields are indistinguishable from the adjacent normal mucosa, the full extent of the cancerized fields cannot be known. Chemoprevention therapy which affects broad oral mucosal surface is an attractive alternative to simple observation, especially for individuals with a cancerized field.
Significant increases in the levels of pEGFR22-24, cyclin D125-27, pStat328-32 and cox-233,34 were detected in cancerized field. Upregulated cox-2 was observed in patients with head and neck cancer as well as in normal epithelium adjacent to tumors34. Bartkova et al.38 observed cyclin D1 expression in sections of normal mucosa adjacent to HNSCC that were not seen in sections of normal mucosa from healthy individuals. Cell cycle deregulation by cyclin D1 induction through the pEGFR signaling pathway is an important event in neoplastic transformation22-27. Subsequently, constitutive activation of pEGFR has been demonstrated in nearly all OSCCs22. Overexpression of pStat3 was also observed in histologically normal tissue adjacent to oral cancer compared to control normal mucosa28.
These molecules overexpressed in the cancerized fields are the molecular targets of EGCG. Assessment of pre and post treatment levels of target molecules allows for measure of EGCG’s modulatory effect and may serve as a surrogate endpoint in clinical trials. EGCG inhibited phosphorylation of EGFR and Stat3 and also downregulated cyclin D1 promoter activity, thereby decreasing cyclin D1 overexpression11-13. By activation of AMP-activated protein kinase, EGCG inhibited cox-2 expression15. The net effect of EGCG is inhibition of the tumor cell growth and induction of apoptosis11-15. When assessed by immunohistochemistry, we found measurable pEGFR and cox-2 expression in oral epithelial cells collected from the cancerized field. There was decrease in expression levels following EGCG treatment for both pEGFR and cox-2. Although not powered to show significance, these results indicate that the EGCG mouthwash can target and modulate pEGFR and cox-2 expression. We encountered technical difficulties in retrieving antigen for the primary antibodies for pStat3 and cyclin D1 assays and their expression could not be assessed. Cellular proliferation index, ki-67, was also measured. ki-67 is expressed in all cells that are not in G0 phase (those in G1, S, G2). Its expression is increased in oral premalignant lesions and the number of ki-67 positive cells correlates with the grade of dysplasia39. In our study, there was decrease in ki-67 positive cells after the 7-day EGCG treatment.
Use of EGCG as a 2-minute ‘swish-and-spit’ mouthwash regime maximizes local exposure while minimizing systemic toxicity. Such agent with minimal adverse event is especially beneficial for a long-term use. Seemingly promising chemopreventive agent retinoid 13-cis-retinoic acid (13cRA) produced a complete clinical and histologic response in 67% of the participants40. However, toxicity was substantial and over half of the responders had recurrence within 3 months of stopping intervention. In comparison, Chow et al. demonstrated the safety of using 800 mg EGCG, p.o., daily for 4 weeks in healthy individuals with only mild adverse events20,37. This dose is equivalent to 8-16 cups of green tea consumption per day, which is similar to daily intake by the heavy tea drinkers. Topical use of EGCG as a mouthwash reduces the risk of systemic toxicity even further. Low toxicity allows for use of this agent for a prolonged period, which may delay future recurrences.
The one week course of daily 800 mg EGCG in an oral suspension formula was indeed well tolerated by the participants. We found no measurable EGCG in plasma, while detectable levels were found in saliva after completion of the study. Lee and his colleagues have shown that after holding 34.5 mg EGCG containing green tea leaves for 2-5 minutes, the total local (saliva) concentration was 2-131 μM and Tmax was 1-10 min41. Considering IC50 of 8-18 μM for both oral precancerous lesions and OSCC16, these concentrations are expected to be within the range achievable in the saliva by holding an 800 mg ECGC oral suspension for 2 minutes. Because increased gastrointestinal absorption was observed with a daily 800 mg bolus dose, compared to a 400 mg twice a daily regime37, we opted to use a solution that delivers a bolus dose of 800 mg EGCG. Moreover, use of the solution before bedtime was expected to minimize potential washout of the study agent by food and drinks.
This feasibility trial is limited in sample size and lacks power to interpret the significance of changes in biomarker expression level. Moreover, this study was not placebo-controlled, in which rinsing with Ora-Blend without EGCG (vehicle control) may have demonstrated the absence of impact on immunoreactivity. Our study, nevertheless, shows feasibility of applying EGCG topically in a form of mouthwash and measuring the modulatory effect of EGCG on its target molecules using proper biomarkers. We were also able to demonstrate that topical application of 800mg EGCG is well absorbed by oral mucosa but systemically undetectable, allowing for minimal systemic toxicity experienced by participants. Future trials will be conducted for an extended period of time with a larger sample size and matching placebo and healthy control arms. A long-term follow-up will further allow for assessment of sustainability of the favorable changes in marker expression level produced by EGCG.
Acknowledgments
In loving memory of I. Bernard Weinstein, MD, DSci.
Grant Support: This work was supported by a K12 Career Development Award (A. Yoon) provided through the Clinical and Translational Science Award KL2 RRO24157 (H. Ginsberg) from the NCRR/NIH, UL1 RR024156 from NCRR/NIH (H. Ginsberg) and NIEHS/NIH grant P30 ES009089 (R. Santella).
References
- 1.Cardesa A, Gale N, Nadal A, Zidar N. Squamous cell carcinoma. In: Barnes L, Eveson JW, Reichar P, Sidransky D, editors. World Health Organization Classification of Tumors, Pathology and Genetics of Head and Neck Tumors. IARC Press; Lyon, France: 2005. pp. 118–121. [Google Scholar]
- 2.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5:311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Damm DD, Allen CM, Bouquot JE. Epithelial pathology. In: Schrefer J, Rudolph P, Alvis K, McKinley L, Forest E, Ramirez J, editors. Oral and Maxillofacial Pathology. Third Edition Saunders; Philadelphia, PA: 2007. pp. 315–388. [Google Scholar]
- 4.Braakhuis BM, Brakenhoff BH, Leemans CR. Second field tumors: a new opportunity for cancer prevention? The Oncologist. 2005;10:493–500. doi: 10.1634/theoncologist.10-7-493. [DOI] [PubMed] [Google Scholar]
- 5.Tabor MP, Brankenhoff RH, Ruijter-Schippers HJ, Kummer JA, Leemans CR, Braakhuis BJ. Genetically altered fields as origin of locally recurrent head and neck cancer: a retrospective study. Clin.Cancer Res. 2004;10:3607–3613. doi: 10.1158/1078-0432.CCR-03-0632. [DOI] [PubMed] [Google Scholar]
- 6.Braakhuis BJ, Tabor MP, Leemans CR, van der Waal I, Snow GB, Brakenhoff RH. Second primary tumors and field cancerization in oral and oropharyngeal cancer: molecular techniques provide new insights and definitions. Head Neck. 2002;24:198–206. doi: 10.1002/hed.10042. [DOI] [PubMed] [Google Scholar]
- 7.Braakhuis BJ, Leemans CR, Brakenhoff RH. A genetic progression model of oral cancer: current evidence and clinical implications. J Oral Pathol Med. 2004;33:317–322. doi: 10.1111/j.1600-0714.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsao AS, Kim ES, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21:857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 10.Dakubo AD, Jakupciak JP, Birch-Machin MA, Parr RL. Clinical implications and utility of field cancerization. Cancer Cell Int. 2007;7:2. doi: 10.1186/1475-2867-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masuda M, Suzui M, Weinstein IB. Effects of Epigallocatechin-3-gallate on Growth, epidermal growth factor receptor signaling pathways, gene expression, and chemosensitivity in human head and neck squamous cell carcinoma cell lines. Clin Cancer Res. 2001;7:4220–4229. [PubMed] [Google Scholar]
- 12.Shimizu M, Deguchi A, Lim JTE, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–2746. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 13.Masuda M, Suzui M, Lim JTE, Weinstein IB. Epigallocatechin-3-gallate inhibits activation of her-2/neu and downstream signaling pathways in human head and neck and breast carcinoma cells. Clin Cancer Res. 2003;9:3486–3491. [PubMed] [Google Scholar]
- 14.Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenon (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 2001;15:2022–2024. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- 15.Hwang JT, Ha J, Park IJ, Lee SK, Baik HW, Kim YM, Park OJ. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Letters. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Khafif A, Schantz SP, Chou TC, Edelstein D, Sacks PG. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin. [DOI] [PubMed]
- 17.Elattar TM, Virji AS. Effect of tea polyphenols on growth of oral squamous carcinioma cells in vitro. Anticancer Res. 2000;20:3459–3465. [PubMed] [Google Scholar]
- 18.Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220:218–224. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarts J, Baker V, Larios E, Chung F. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res. 2005;49:43–51. doi: 10.1002/mnfr.200400031. [DOI] [PubMed] [Google Scholar]
- 20.Chow HS, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Ablerts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and Polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- 21.Yoon AJ, Shen J, Wu H, Angelopoulos D, Singer S, Chen R, Santella RM. Expression of Checkpoint Kinase 2 and Histone 2AX in Exfoliative Oral Cells Following Exposure to Ionizing Radiation. Radiat Res. 2009;171:771–775. doi: 10.1667/RR1560.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiraishi Y, Wada T, Nakatani K, Negoro K, Fujita S. Immunohistochemical expression of EGFR and p-EGFR in oral squamous cell carcinomas. Pathol Oncology Res. 2006;12:87–91. doi: 10.1007/BF02893450. [DOI] [PubMed] [Google Scholar]
- 23.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 2001;85:473–483. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- 25.Liu SC, Hu Y, Sute ER, Clapper ML, Chen SY, Lanfranchi HE, Engstrom PF, Klein-Szanto AJ. Image analysis of p53 and cyclin D1 expression in premalignant lesions of the oral mucosa. Anal Quant Cytol Histol. 1999;21:166–173. [PubMed] [Google Scholar]
- 26.Opitz OG, Suliman Y, Hahn WC, Harada H, Blum HE, Rustgi AK. Cyclin D1 overexpression and p53 inactivation immortalize primary oral keratinocytes by a telomerase-independent mechanism. J Clin Invest. 2001;108:725–732. doi: 10.1172/JCI11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu SC, Sauter ER, Clapper ML, Feldman RS, Levin L, Chen SY, Yen TJ, Ross E, Engstrom PF, Klein-Szanto AJ. Markers of cell proliferation in normal epithelia and dysplastic leukoplakias of the oral cavity. Cancer Epidemiol Biomarkers Prev. 1998;7:597–603. [PubMed] [Google Scholar]
- 28.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, Yukai H, Kim JD. Constitutive activation of Stat 3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. PNAS. 2000;97:4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, Tomita K, Komiyama S, Weinstein IB. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 30.Xi S, Zhang Q, Dyer KF, Lerner EC, Smithgall TE, Gooding WE, Kamens J, Grandis JR. Src kinase mediate STAT growth pathways in squamous cell carcinoma of the head and neck. J Biol Chem. 2003;278:31574–31583. doi: 10.1074/jbc.M303499200. [DOI] [PubMed] [Google Scholar]
- 31.Song JI, Grandis JR. STAT signaling in head and neck cancer. Oncogene. 2000;19:2489–2495. doi: 10.1038/sj.onc.1203483. [DOI] [PubMed] [Google Scholar]
- 32.Lee TL, Yeh J, Waes CV, Chen Z. Epigenetic modification of SOCS-1 differentially regulates STAT3 activation in response to interleukin-6 receptor and epidermal growth factor receptor signaling through JAK and /or MEK in head and neck squamous cell carcinomas. Mol Cancer Ther. 2006;5(1):8–19. doi: 10.1158/1535-7163.MCT-05-0069. [DOI] [PubMed] [Google Scholar]
- 33.Sawhney M, Rohtgi N, Kaur J, Shishodia S, Sethi G, Gupta SD, Deo SVS, Shukla NK, Aggarwal BB, Ralhan R. Expression of NF-κB parallels COX-2 expression in oral precancer and cancer: association with smokeless tobacco. Int J Cancer. 2007;120:2545–2556. doi: 10.1002/ijc.22657. [DOI] [PubMed] [Google Scholar]
- 34.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, Masferrer JL, Dannenberg AJ. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59:991–994. [PubMed] [Google Scholar]
- 35.Yoon AJ, Shen J, Santella RM, Zegarelli DJ, Chen R, Weinstein IB. Activated checkpoint kinase 2 expression and risk for oral squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16(12):2768–2772. doi: 10.1158/1055-9965.EPI-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal R, Katiyar SK, Zaidi SIA, Mukhtar H. Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by pholyphenolic fraction isolated from green tea and its individual epicatechin derivative. Cancer Res. 1992;52:3582–3588. [PubMed] [Google Scholar]
- 37.Chow HS, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, Alberts DS. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Cancer Prev. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 38.Bartkova J, Lukas J, Muller H, Strauss M, Gusterson B, Barteck J. Abnormal patterns of D-type cyclin expression and G1 regulation in human head and neck cancer. Cancer Res. 1995;55:949–956. [PubMed] [Google Scholar]
- 39.Schoelch ML, Regezi JA, Dekker NP, Ng IO, McMillan A, Ziober BL, Le OT, Silverman S, Fu KK. Cell cycle proteins and the development of oral squamous cell carcinoma. Oral Oncol. 1999;35:333–342. doi: 10.1016/s1368-8375(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 40.Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23:346–356. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- 41.Lee MJ, Lambert JD, Prabhu S, Meng X, Lu H, Maliakal P, Ho CT, Yang CS. Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract. Cancer Epi Biomarker Prev. 2004;13:132–137. doi: 10.1158/1055-9965.epi-03-0040. [DOI] [PubMed] [Google Scholar]