Abstract
Objectives. To determine whether molecular remission defined by a multi-biomarker disease activity (MBDA) score predicts a reduced risk of joint damage progression, and whether the MBDA score can augment existing classifications of remission.
Methods. The study examined 271 visits for 163 RA patients in the Leiden Early Arthritis Cohort. The MBDA score and other variables from each visit were evaluated for prediction of progression [change in Sharp–van der Heijde Score (ΔSHS) >3] over the ensuing 12 months. Positive likelihood ratios (PLRs) for non-progression were calculated for remission based upon DAS based on 28-joint counts and CRP (DAS28-CRP <2.32), EULAR/ACR Boolean criteria and MBDA score (≤25).
Results. Ninety-three per cent of patients in MBDA-defined remission did not experience progression, compared with 70% of patients not in MBDA remission (P = 0.001). There were no significant differences in the fraction of non-progressers between patients in remission and those not in remission using either DAS28-CRP or EULAR/ACR criteria. The PLR for non-progression over 12 months for MBDA remission was 4.73 (95% CI 1.67, 15.0). Among patients in DAS28-CRP remission, those with a high MBDA score were 2.3 times as likely (95% CI 1.1, 3.7) to have joint damage progression during the next year.
Conclusion. MBDA-defined remission was an indicator of limited radiographic progression over the following 12 months. For patients in DAS28-CRP remission, high MBDA scores were a significant indicator of elevated risk of progression. MBDA results may provide a useful adjunct to clinical assessment to identify progression-free remission and assess subclinical disease.
Keywords: biomarkers, rheumatoid arthritis, remission, EAC, radiographic progression
Introduction
RA is a chronic inflammatory disease that principally attacks the synovial joints, resulting in joint damage, physical disability and premature mortality [1–4]. There is no known cure for RA, and hence a primary aim in the treatment of RA is to achieve a state of remission [5–7]. Identifying patients in true remission has been a challenge, however, despite the development of an assortment of definitions [8].
To address this issue, a joint committee of the ACR and the European League Against Rheumatism (EULAR) has made suggestions for defining remission in clinical practice and has proposed consensus definitions of remission for use as outcome measures in clinical trials [9]. The recommendations of the joint committee were based on a systematic review of the prognostic validity of current remission definitions [10] and the goal was to create stringent definitions of remission that would predict good functional outcomes and the absence of radiographic progression [11]. Although excluded from the consensus definitions, measures of physical function and radiographic damage were used to validate the candidate remission definitions. These measures are particularly important since studies have shown that RA patients who meet established criteria for clinical remission may nonetheless experience progressive structural damage [12–17].
We recently developed and validated a multi-biomarker disease activity (MBDA) blood test that could be valuable in the management of patients with RA [18]. The MBDA test employs an algorithm to combine the levels of 12 serum biomarkers into a single score from 1 to 100 to provide an objective measure of RA disease activity. A score of ≤25 indicates remission, whereas scores falling into the ranges 26–29, 30–44 and >44 indicate low, moderate and high RA disease activity, respectively. The MBDA test is intended to be used in conjunction with existing symptom-based disease activity measures with the objective of improving long-term outcomes for RA patients. Since the biomarkers can reflect the activity of biological pathways underlying the disease, low MBDA scores might define a state of molecular remission indicative of true disease quiescence [19].
In the present study we sought to examine the relationship between three different definitions of RA remission and radiographic progression in early RA patients. Remission was defined using the MBDA score, the ACR/EULAR Boolean-based consensus definition [9] or the modified DAS based on 28-joint counts and CRP (DAS28-CRP) [20]. Our aims were to compare the frequency of joint damage progression observed in remission and non-remission patients for each of the three remission definitions and to determine whether the MBDA test provides information about the risk of progression that is complementary to established clinical definitions of remission.
Methods
Patient population
The study population was selected from the Leiden Early Arthritis Clinic (EAC) cohort, a group of patients with recent-onset arthritis who were enrolled at the Department of Rheumatology of Leiden University Medical Center beginning in 1993 [21]. Enrollees in the EAC cohort had a symptom duration of <2 years and fulfilled the ACR 1987 revised criteria for the classification of RA [22]. Follow-up visits were performed yearly and the median time between enrolment in EAC and the first study visit included in this analysis was 4.6 years. Subject visits that were eligible for inclusion in the analysis had available serum samples for biomarker analysis, clinical data enabling calculation of the 28-joint DAS, Sharp–van der Heijde score (SHS) and an additional SHS 1 year later to allow assessment of radiographic progression. These eligible subject visits were sampled with the intention of obtaining a study population in which ∼60% of patients were positive for anti-CCP and ∼40% of patients had a change in DAS of at least 1.2 over 12 months. The intended proportion of anti-CCP-positive patients is representative of early RA populations [23] and of early RA in the Leiden EAC. The criterion related to the change in DAS was added to facilitate analysis of changes in disease activity for work that is outside the scope of this article. In this cohort, the correlation between 1-year change in DAS and change in SHS (ΔSHS) over the same year was extremely weak, with r = −0.11 (R2 = 0.01; n = 98 subjects with complete data). Because of the weakness of this correlation, the employed sampling mechanism should have negligible impact on the analyses of radiographic progression in this article. Subject visits for this study were selected without any knowledge of biomarker concentrations or changes in SHS. The patients studied here are representative of the set of RA patients included in the Leiden EAC in the concomitant time period. Informed consent was obtained for all patients and the study was approved by the local ethics committee for the Leiden University Medical Center.
Sample size
The sample size of 271 visits was chosen to enable detection of associations between biomarker-based measures of disease activity and clinical variables. The probability of detecting a significant difference between a group with a true event rate of 50% and a group with a true event rate of 30% with 135 visits per group (270 total visits) at an alpha level of 0.05 is >90%.
Radiographic assessment
Radiographic damage in the hands and feet was scored chronologically according to the Sharp–van der Heijde method [24] by one experienced reader. Four hundred and ninety-nine randomly selected X-rays were scored twice, and the within-reader (intraclass) correlation coefficient (ICC) was 0.91. The scorer was blinded to the clinical data and was not aware of the study questions [21].
Radiographic progression
Radiographic progression was quantified using the ΔSHS over the 12 months following sample collection and clinical assessment [25]. In the primary analyses, patients with a 12-month ΔSHS ≤3 were classified as non-progressors. Supplemental analyses were performed using ΔSHS cut-off values of 0 and 5 to define non-progression. The performance of the various remission criteria was assessed using three different ΔSHS cut-offs in order to evaluate the robustness of the results and conclusions of the study across different definitions of radiographic progression.
MBDA score
MBDA scores were generated using the same platform, reagents and algorithm as the Vectra DA test (Crescendo Bioscience, Inc., South San Francisco, CA, USA), which combines the concentrations of vascular cell adhesion molecule-1, epidermal growth factor, VEGF-A, IL-6, TNF-receptor 1 (TNF-RI), MMP-1, MMP-3, cartilage glycoprotein-39 (YKL-40), leptin, resistin, SAA and CRP in a pre-specified algorithm to generate a score between 1 and 100 [26]. Quantification of the 12 biomarkers was performed with sandwich assays in three separate multiplex panels and the MBDA score was calculated as a multi-linear combination of analyte concentrations [27]. A pre-specified algorithm was applied in which measured serum concentrations from the Leiden samples were used with optimized weights to estimate the 28-joint tender joint count (TJC28), 28-joint swollen joint count (SJC28) and patient global (PG) health score. Different subsets of biomarkers were used to estimate the TJC28, SJC28 and PG. The biomarkers and weightings used to predict these components of the DAS28-CRP were previously determined and validated in a multi-step process in independent cohorts. Lastly, predicted values for the TJC, SJC and PG were combined with the CRP concentration in a formula analogous to the equation for the DAS28-CRP and then converted to a score from 1 to 100. For this study, modifications were made to the information systems and review procedures as follows: data were captured in a database that is different from the one used for reporting clinical patient results, and quality control of assay results included multi-plate, experiment-wise consistency checks that are not built into the plate-wise quality control of the clinical reporting system.
Disease activity was categorized using the MBDA score as follows: >44 (high), 30–44 (moderate) and 26–29 (low). Patients with MBDA scores ≤25 were considered to be in molecular remission as defined by the MBDA test.
DAS28-CRP
DAS28-CRP scores were derived from the TJC28 and SJC28, the patient’s general health status measured with a visual analogue scale and the CRP concentration (in mg/l) [5]. The DAS28-CRP scores for each patient were calculated using the following formula from the DAS website (http://www.das-score.nl/):
Disease activity based on the DAS28-CRP was classified as high (DAS28-CRP >4.09), moderate (DAS28-CRP 2.67–4.09) or low (DAS28-CRP 2.32–2.67) and patients with DAS28-CRP scores <2.32 were considered to be in remission according to the adjusted thresholds described by Inoue et al. [28].
ACR/EULAR score
The Boolean-based definition of remission proposed by the ACR/EULAR committee was determined as described [9]. Patients were considered to be in remission by the ACR/EULAR criteria if they satisfied all of the following: TJC28 ≤1, SJC28 ≤1, CRP ≤1 mg/dl and PG assessment ≤1 (on a 0–10 scale).
Statistical analysis
The pre-test and post-test odds of progression were calculated for definitions of remission based on the DAS28-CRP score, the ACR/EULAR Boolean criteria and the MBDA score. Pre-test odds were calculated as the total number of non-progressors divided by the total number of progressors. Post-test odds were calculated as the number of non-progressors who met the corresponding set of remission criteria divided by the number of progressors who met the remission criteria. Definitions of remission were evaluated using the positive likelihood ratio (PLR), where the PLR is the ratio between the post-test and pre-test odds of non-progression [29].
The incremental value of the MBDA test was assessed by calculating the relative risk (RR) [30] of radiographic progression in the subset of patients with high MBDA scores and DAS28-CRP scores indicating remission vs all patients in remission as defined by the DAS28-CRP. This nested analysis provides an answer to the question: for patients who are known to be in DAS28-remission, does the prognosis change if they are found to have a high MBDA score?
To evaluate the relationship between disease activity and radiographic progression, the RR was calculated as the percentage of patients with high disease activity who experienced radiographic progression divided by the percentage of patients in remission who experienced radiographic progression.
CIs and associated P-values for PLRs and RRs were derived using bootstrap resampling [31]. This methodology relaxes the need for assumptions of normality and facilitates nested analysis by taking account of the overlap between groups. P-values for differences in the proportion of good outcomes between patients in remission and those not in remission were calculated using Fisher’s exact test. All statistical calculations were performed using the package R (www.r-project.org).
Results
A total of 271 study visits were evaluated for 163 subjects with recent-onset arthritis. A summary of the clinical characteristics of the study population is provided in Table 1, along with corresponding characteristics for the overall EAC cohort. The median SHS at entry into the EAC was 6, with an interquartile range (IQR) over the 25th–75th percentile of 2–11 in the analysis data set, vs a median SHS of 5 (IQR 2–12) in the unanalysed patients from the EAC. During the time between enrolment in the EAC and the first study visit in the analysis, the median SHS increased to 23 (IQR 11–47). None of the patients was receiving anti-TNF therapy at the baseline visit in the analysis data set. During follow-up the registered frequency of anti-TNF use was <5%. Patients in the study were treated with D-Pen, HCQ, SSZ or MTX. Because of the infrequent use of anti-TNF therapies in this cohort, with most patient visits occurring in the 1990s, these data may be more representative of the natural course of RA than data obtained in the post-2000 biologic era.
Table1.
Clinical characteristics of the study population
| Characteristica | Patients analysed | Unanalysed patients in parent EAC population |
|---|---|---|
| Unique subjects, n | 163 | 809 |
| Gender, female, % | 67 | 67 |
| Age, mean (s.d.) | 55 (14) | 57 (16) |
| RF positive, % | 65 | 55 |
| Anti-CCP positive, % | 66 | 50 |
| Inclusion year, mean (min–max) | 1998 (1993–2004) | 2003 (1993–2010) |
aAge, gender and rates of anti-CCP and RF positivity were assessed at the time of enrolment into the Early Arthritis Cohort. min–max: minimum–maximum.
Overall, the patients in the analysis appeared to be representative of those in the larger EAC population. The increased proportion of patients who were anti-CCP positive or RF positive in the analysis data set is consistent with the observation that analysed patients were enrolled relatively early in the course of the EAC study and that rates of anti-CCP and RF positivity in the EAC have dropped somewhat over time.
Risk of non-progression using different definitions of remission
A comparison of the rates of radiographic non-progression (ΔSHS ≤ 3) for patients meeting each of the three sets of remission criteria—DAS28-CRP < 2.32, ACR/EULAR (TJC28, SJC28, PG, CRP ≤ 1) and MBDA ≤ 25—is given in Table 2. Eighty-three patients met the definition of remission based upon DAS28-CRP, 43 patients were in MBDA-defined remission and 30 patients met the ACR/EULAR remission criteria. Patients in MBDA-defined remission had significantly greater rates of non-progression than patients who did not meet the MBDA remission criterion (P = 0.001). Similar results were observed for ΔSHS cut-off values of 0 and 5 (supplementary Table S1, available as supplementary data at Rheumatology Online). There were no significant differences in the rate of non-progression between patients in remission and those not in remission for DAS28-CRP and ACR/EULAR.
Table 2.
Comparison of remission and non-remission groups using a 12-month radiographic non-progression threshold of ΔSHS ≤ 3 (n = 271 total visits)
| Percentage of patients with good radiographic outcome (n/n) |
|||
|---|---|---|---|
| Test for remission | Remission group | Non-remission group | P |
| MBDA ≤ 25 | 93 (40/43) | 70 (160/228) | 0.001 |
| DAS28-CRP < 2.32 | 80 (66/83) | 71 (134/188) | 0.18 |
| ACR/EULARa | 83 (25/30) | 73 (175/241) | 0.27 |
aACR/EULAR remission criteria were TJC28, SJC28, PG and CRP ≤ 1.
The PLRs associated with each definition of remission for identifying patients that did not progress [9] are shown in Table 3. The PLRs were 4.73, 1.78 and 1.38 for remission defined by MBDA score, ACR/EULAR criteria and DAS28-CRP, respectively. Only the PLR for MBDA score remission was significantly greater than 1 (P = 0.001). Similar patterns were observed for other ΔSHS cut-offs, and MBDA score remission was also the only remission definition with a PLR significantly greater than 1 with a ΔSHS cut-off of 5 (supplementary Table S2, available as supplementary data at Rheumatology Online). None of the PLRs for the three definitions of remission was significantly greater than 1 when a ΔSHS cut-off value of 0 was used.
Table 3.
Comparisons of positive likelihood ratios using a 12-month radiographic non-progression threshold of ΔSHS ≤ 3 (n = 271 total visits)
| Odds of non-progression |
||||
|---|---|---|---|---|
| Test for remission | Pre-test | Post-test | PLR (ratio of post-test to pre-test odds) | 95% CI |
| MBDA ≤ 25 | 2.82 (200/71) | 13.3 (40/3) | 4.73 | 1.67, 15.0 |
| DAS28-CRP < 2.32 | 2.82 (200/71) | 3.88 (66/17) | 1.38 | 0.90, 2.38 |
| ACR/EULARa | 2.82 (200/71) | 5.00 (25/5) | 1.78 | 0.72, 5.17 |
aACR/EULAR remission criteria were TJC28, SJC28, PG and CRP ≤ 1.
Risk of radiographic progression and different measures of disease activity
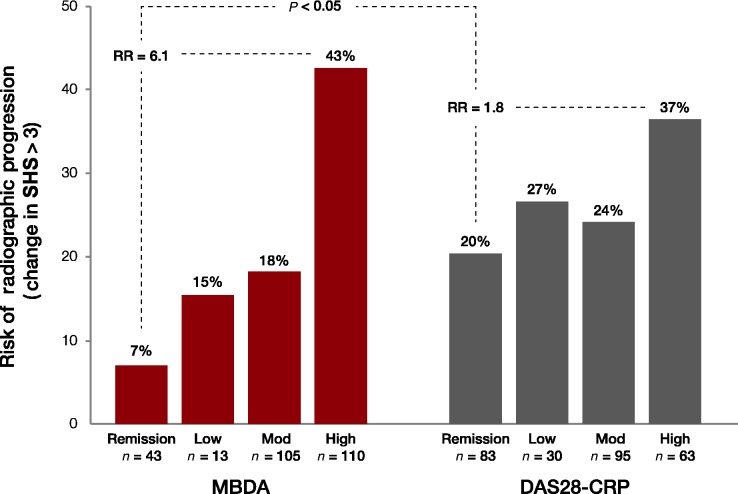
The relationship between risk of radiographic progression and disease activity as measured by the MBDA and the DAS28-CRP is shown in Fig. 1. Among the 43 subjects who met the MBDA remission criterion (MBDA ≤ 25), only 3 (7%) experienced radiographic progression over the ensuing 12 months. The risk of radiographic progression increased steadily with increasing disease activity as defined by MBDA, from subjects with low disease activity (2/13, 15% risk) to moderate (19/105, 18%) to high (47/110, 43%). An assessment of RR showed that subjects with high MBDA scores were six times more likely to experience an increase in SHS as those in remission as defined by the MBDA (RR = 43%/7% = 6.1).
Fig. 1.
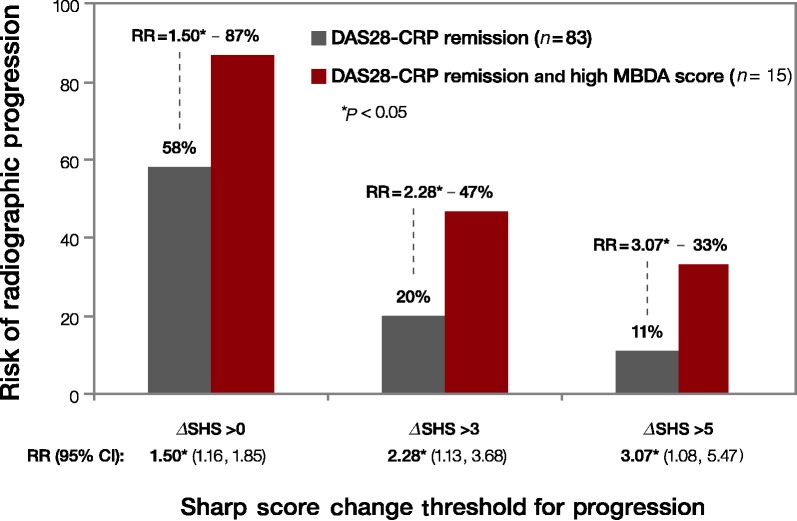
A high MBDA score for subjects in DAS28-CRP remission indicates increased risk of radiographic progression.
The risk of radiographic progression also increased with disease activity classification as assessed by the DAS28-CRP, from remission (17/83, 20% risk) to low (8/30, 27%) to moderate (23/95, 24%) to high (23/63, 37%). However, subjects with high DAS28-CRP values were only twice as likely to experience radiographic progression as those in DAS28-CRP remission (RR = 37%/20% = 1.8). The increase in the risk of radiographic progression for patients with high disease activity relative to those in remission was significantly greater for MBDA than for DAS28-CRP (RR = 6.1 vs RR = 1.8, P < 0.05).
Risk of radiographic progression among subjects in DAS28-CRP remission
Among the 83 subjects in this study who met the DAS28-CRP remission criteria (DAS28-CRP < 2.32), a subset of 15 subjects also had a high MBDA score (MBDA > 44). Figure 2 shows the risk of radiographic progression for all subjects with a DAS28-CRP < 2.32 vs the subset of subjects with high MBDA scores. Twenty per cent (17/83) of subjects who met the DAS28-CRP remission criteria experienced radiographic progression (ΔSHS > 3) over the ensuing 12 months. By comparison, 7 of 15 (47%) subjects who both met the DAS28-CRP remission criteria and had high MBDA scores experienced radiographic progression. Thus a subject in DAS28-CRP remission was more than twice as likely to experience progression if he/she also had a high MBDA score (RR = 47%/20% = 2.28; 95% CI 1.13, 3.68). Using different ΔSHS cut-offs for defining radiographic progression (0, 3 or 5) produced different estimates of RR, but in all cases the risk of progression for subjects who met the DAS28-CRP remission criteria was significantly greater for those who also had high MBDA scores.
Fig. 2.
Risk of radiographic progression vs level of disease activity.
Among the 30 subjects in this study who met the ACR/EULAR remission criteria, 2 subjects also had a high MBDA score. Both of these subjects experienced radiographic progression (one subject had a ΔSHS of 3, the other had a ΔSHS of 8). By comparison, the median ΔSHS was equal to 1 for the other 28 subjects in ACR/EULAR remission. Because of the small number of subjects in ACR/EULAR remission with high MBDA scores, it was not possible to perform a formal analysis of RR.
Discussion
Reliable assessment of remission is important for the optimal management of patients with RA. Both composite indices such as the DAS28-CRP and the new remission criteria proposed by the ACR/EULAR committee rely on clinical evaluation of tender and swollen joints in the assessment of remission among patients with RA [9]. However, recent studies have shown that these clinical measures are not sufficiently sensitive for classifying remission since they cannot exclude the presence of active disease as evidenced by imaging-detected inflammation or radiological progression [15, 32]. For example, radiographic progression was observed during a 2-year follow-up period for many patients in the Swedish Pharmacotherapy (SWEFOT) trial who had responded well clinically (DAS28 < 3.2) to initial treatment with MTX [33]. Other studies have shown that the majority of patients in drug-assisted remission had active inflammation, whether the administered therapy was a DMARD or a biological agent [13, 34]. These findings indicate that patients can have subclinical disease despite displaying few clinical signs and symptoms, and illustrate the importance of identifying new measures to supplement traditional symptom-focused evaluations in the assessment of remission status. Using subjects from the Leiden Early Arthritis Cohort, this study evaluated the relationships between three different definitions of remission and radiographic progression. It was shown that MBDA-defined remission was a significant predictor of radiographic non-progression, whereas remission defined by traditional DAS28-CRP or ACR/EULAR criteria was not. Moreover, the MBDA test was useful in assessing the risk of radiographic progression among patients who met clinical remission criteria—subjects with DAS28-CRP scores <2.32 were more than twice as likely to experience radiographic progression if they also had an MBDA score >44.
Several limitations should be taken into account when interpreting the results from this study. First, it focused on patients with RA relatively early in the disease and did not include patients with long duration of disease. Secondly, the population selected for this study had a higher rate of progression than many other cohorts, particularly those with greater use of biologic therapies. Since the inferences of the analysis are based on the RRs of progression we would expect the same trends (in terms of increased or decreased risk) to hold true in other patient populations. However, further studies are required to determine the magnitude of the risks observed in cohorts with different patient characteristics. Thirdly, we did not evaluate remission definitions based on Clinical Disease Activity Index (CDAI) or Simplified Disease Activity Index (SDAI) because the physician global assessment, which is required for the calculation of these indices, was not available. These two measures of disease activity are commonly used and should be included in future studies. Finally, the small numbers of patients in some categories (e.g. in clinical remission but with high MBDA scores) limit our ability to address some questions of interest. It will be important to determine whether the MBDA test can identify patients at high risk of radiographic progression in larger cohorts of patients with early as well as long-duration RA since there is evidence to suggest that patients in the latter group may be more recalcitrant to treatment. It will also be important to evaluate the MBDA test together with clinical criteria in trials focusing on the achievement of remission.
In conclusion, patients in molecular remission by MBDA have favourable radiographic outcomes, and the MBDA score provides complementary information to clinical assessments of remission. Further studies are needed to enable better predictions of radiographic progression in patients who meet the criteria for clinical or molecular remission.

Supplementary Material
Acknowledgements
The authors would like to thank all the individuals who participated in this study. They also thank Bill Manning and Ferhan Qureshi for ensuring the quality of the assay results used in the study, Paula Adduci for graphics and Linda Kahl, a consultant with Crescendo Bioscience, for assistance with manuscript preparation.
Funding: The work of A.H.M.vdH.-vM. is supported by the Netherlands Organization for Health Research and Development. The work of R.K. is supported by the Dutch Arthritis Association. Crescendo funded the generation of biomarker data and the statistical analysis.
Crescendo provided funds to the Leiden University Medical Center for the retrieval, aliquotting, labelling and shipping of study samples to Crescendo’s laboratory facility. No other funding was received for the study.
Disclosure statement: T.W.J.H. serves on the scientific advisory board of Crescendo. D.J.H. and G.C. are employees of and hold stock options in Crescendo Bioscience. All other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1.Mitchell DM, Spitz PW, Young DY, et al. Survival, prognosis, and causes of death in rheumatoid arthritis. Arthritis Rheum. 1986;29:706–14. doi: 10.1002/art.1780290602. [DOI] [PubMed] [Google Scholar]
- 2.Pincus T, Callahan LF, Sale WG, et al. Severe functional declines, work disability, and increased mortality in seventy-five rheumatoid arthritis patients studied over nine years. Arthritis Rheum. 1984;27:864–72. doi: 10.1002/art.1780270805. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F. The natural history of rheumatoid arthritis. J Rheumatol Suppl. 1996;44:13–22. [PubMed] [Google Scholar]
- 4.Yelin E, Trupin L, Wong B, Rush S. The impact of functional status and change in functional status on mortality over 18 years among persons with rheumatoid arthritis. J Rheumatol. 2002;29:1851–7. [PubMed] [Google Scholar]
- 5.ACR Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 update. Arthritis Rheum. 2002;46:328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 6.Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–7. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sokka T, Hannonen P, Makinen H. Remission: a realistic goal in rheumatoid arthritis? Int J Clin Rheumatol. 2011;6:643–7. [Google Scholar]
- 9.Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European League Against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404–13. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 10.van Tuyl LH, Felson DT, Wells G, et al. Evidence for predictive validity of remission on long-term outcome in rheumatoid arthritis: a systematic review. Arthritis Care Res. 2010;62:108–17. doi: 10.1002/acr.20021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Tuyl LH, Vlad SC, Felson DT, Wells G, Boers M. Defining remission in rheumatoid arthritis: results of an initial American College of Rheumatology/European League Against Rheumatism consensus conference. Arthritis Rheum. 2009;61:704–10. doi: 10.1002/art.24392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008;58:2958–67. doi: 10.1002/art.23945. [DOI] [PubMed] [Google Scholar]
- 13.Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease-modifying antirheumatic drug-induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum. 2006;54:3761–73. doi: 10.1002/art.22190. [DOI] [PubMed] [Google Scholar]
- 14.Cohen G, Gossec L, Dougados M, et al. Radiological damage in patients with rheumatoid arthritis on sustained remission. Ann Rheum Dis. 2007;66:358–63. doi: 10.1136/ard.2006.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lillegraven S, Prince FH, Shadick NA, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis. 2012;71:681–6. doi: 10.1136/ard.2011.154625. [DOI] [PubMed] [Google Scholar]
- 16.Molenaar ET, Voskuyl AE, Dinant HJ, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum. 2004;50:36–42. doi: 10.1002/art.11481. [DOI] [PubMed] [Google Scholar]
- 17.Salaffi F, Carotti M, Ciapetti A, et al. Relationship between time-integrated disease activity estimated by DAS28-CRP and radiographic progression of anatomical damage in patients with early rheumatoid arthritis. BMC Musculoskelet Disord. 2011;12:120. doi: 10.1186/1471-2474-12-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bakker MF, Cavet G, Jacobs JW, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis. 2012;71:1692–7. doi: 10.1136/annrheumdis-2011-200963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–19. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 20.Prevoo ML, van 't Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 21.de Rooy DP, van der Linden MP, Knevel R, Huizinga TW, van der Helm-van Mil AH. Predicting arthritis outcomes—what can be learned from the Leiden Early Arthritis Clinic? Rheumatology. 2011;50:93–100. doi: 10.1093/rheumatology/keq230. [DOI] [PubMed] [Google Scholar]
- 22.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 23.Riedemann JP, Munoz S, Kavanaugh A. The use of second generation anti-CCP antibody (anti-CCP2) testing in rheumatoid arthritis–a systematic review. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S69–76. [PubMed] [Google Scholar]
- 24.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27:261–263. [PubMed] [Google Scholar]
- 25.van der Heijde D, Boers M, Lassere M. Methodological issues in radiographic scoring methods in rheumatoid arthritis. J Rheumatol. 1999;26:726–30. [PubMed] [Google Scholar]
- 26.Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multi-biomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res. 2012 doi: 10.1002/acr.21767. Advance Access published 26 June 2012, doi:10.1002/acr.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eastman PS, Manning WC, Qureshi F, et al. Characterization of a multiplex, 12-biomarker test for rheumatoid arthritis. J Pharm Biomed Anal. 2012;70:415–24. doi: 10.1016/j.jpba.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Inoue E, Yamanaka H, Hara M, Tomatsu T, Kamatani N. Comparison of disease activity score (DAS)28- erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis. 2007;66:407–9. doi: 10.1136/ard.2006.054205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deeks JJ, Altman DG. Diagnostic tests 4: likelihood ratios. BMJ. 2004;329:168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armitage P, Berry G, Matthews JNS. Oxford: Blackwell Science; 2002. Statistical methods in medical research. [Google Scholar]
- 31.Efron B. Better bootstrap confidence intervals. J Am Stat Assoc. 1987;82:171–85. [Google Scholar]
- 32.Klarenbeek NB, Koevoets R, van der Heijde DM, et al. Association with joint damage and physical functioning of nine composite indices and the 2011 ACR/EULAR remission criteria in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1815–21. doi: 10.1136/ard.2010.149260. [DOI] [PubMed] [Google Scholar]
- 33.Rezaei H, Saevarsdottir S, Forslind K, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2-year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis. 2012;71:186–91. doi: 10.1136/annrheumdis-2011-200038. [DOI] [PubMed] [Google Scholar]
- 34.Saleem B, Brown AK, Keen H, et al. Disease remission state in patients treated with the combination of tumor necrosis factor blockade and methotrexate or with disease-modifying antirheumatic drugs: a clinical and imaging comparative study. Arthritis Rheum. 2009;60:1915–22. doi: 10.1002/art.24596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.