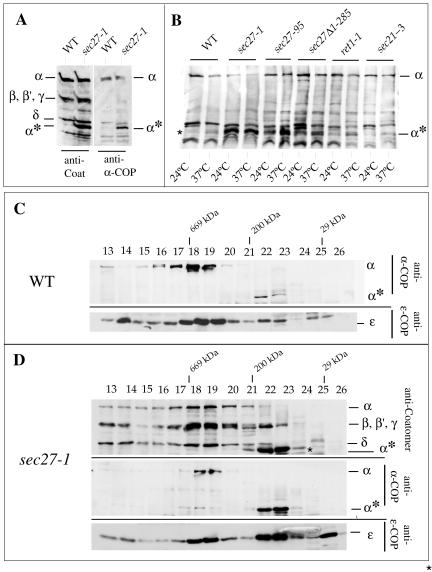
Figure 4.
α-COP is destabilized in sec27-1 cells. (A and B) Identification of a ∼65-kDa α-COP fragment (α*) in sec27-1 cells and other coatomer mutants as indicated, and in wild-type cells (WT). For details, see text. (C) Superose 6 gel filtration of cytosolic proteins from temperature-shifted wild-type cells (WT). α-COP species were identified with the α-COP peptide antiserum, and ε-COP with a specific rabbit antiserum. (D) Superose 6 gel filtration of cytosolic proteins from sec27-1 cells. SDS-PAGE separated proteins of column fractions were probed with anti-coatomer antiserum to identify α-, β′-, β-, and γ-COP and the α-COP fragment (α*) (top). The antiserum against the carboxy-terminal α-COP peptide (“anti-α-COP”) was used (middle); the bottom panel visualizes ε-COP. Note the reduced signal for intact α-COP in fractions 18 + 19 from sec27-1 cells compared with wild type. Further note that a large fraction of α-COP immunoreactivity is present in fractions 22 + 23, corresponding to a partial complex of ∼200 kDa in which ε-COP cofractionates. An additional peak of monomeric ε-COP is seen in fraction 26. Fraction numbers and positions of marker proteins are indicated: thyroglobulin (669,000), β-amylase (200,000), and carboanhydrase (29,000).