Figure 6.
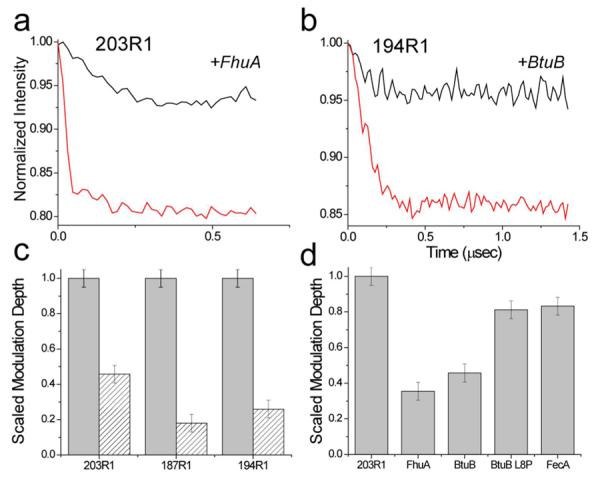
The binding of TonBΔTMD to transporter dissociates the TonB dimer. Dipolar evolution data for two spin-labeled TonBΔTMD mutants: a) 203R1 and b) 194R1, when bound to the transporters FhuA or BtuB in the presence of substrate. For these examples, the data were recorded at X-band, and the pulse excitation parameters were not varied, allowing a comparison of TonBΔTMD oligomerization. Binding of TonBΔTMD to the transporter dramatically reduces the modulation depth in these DEER signals, indicating a loss of dipolar-coupled spins and a dissociation of the TonB oligomer (see Text). In c) the changes in the scaled modulation depth are plotted are plotted for three TonBΔTMD spin labels, 203R1, 197R1 and 194R1 in the absence (grey bars) and presence (hatched bars) of BtuB. In d) the modulation depth of 203R1 when bound to FhuA, BtuB, BtuB L8P, and FecA is compared. The conversion to monomer is less efficient for transporters or mutants that do not exhibit high-affinity TonB binding. Under the conditions of this experiment all added transporter (which was stoichiometrically limiting) was bound to TonB.
