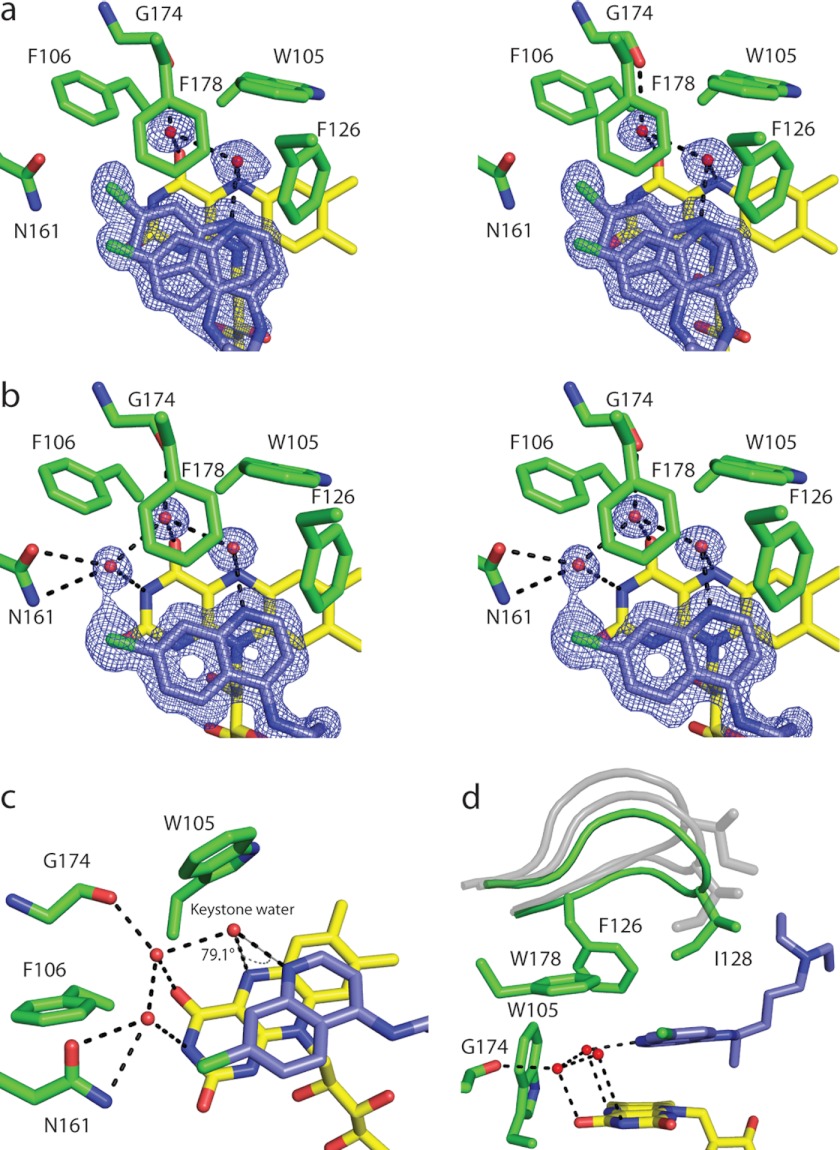
FIGURE 3.
Binding of chloroquine to reduced NQO2. a and b, stereodiagrams illustrating the binding of CQ to the NQO2red active sites in the two dimers of the asymmetric unit; the electron density corresponds to 2Fo − Fc maps contoured at 1σ around CQ. a, the electron density for AB dimer. Shown is the active site of the A subunit, with CQ bound in two alternate positions, one of which corresponds to the position observed in the C and D subunits, whereas the second is shifted slightly. The electron density and positions of bound CQ were similar for the B subunit. b, the electron density and position of bound CQ in the CD dimer. Shown is the active site of the C subunit; the electron density in the D subunit was similar, and for both subunits, CQ was modeled in the same single position. c, the hydrogen bonding network, with the “keystone water” bridging N4 of CQ and N5 of the FAD isoalloxazine ring. d, the active site loop (residues 126–136) is shown in the fully closed conformation (green) observed in NQO2red-CQ; in this state, residue Ile128 makes van der Waals contact with CQ. For reference, the conformations of the loop observed in crystals of oxidized NQO2 are shown in gray. One-letter amino acid codes are used.