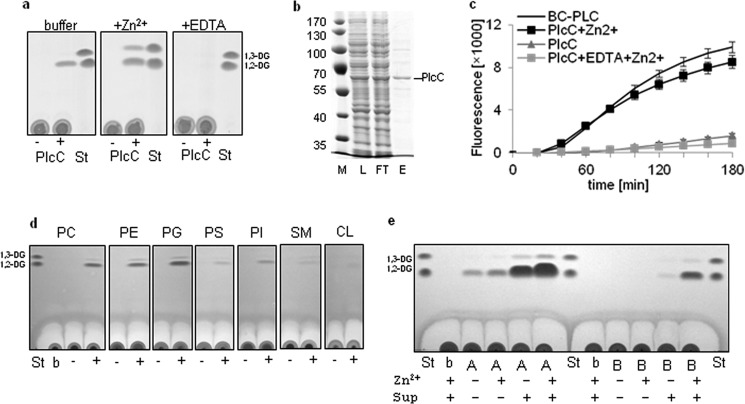
FIGURE 1.
L. pneumophila PlcC exhibits Zn2+-dependent PLC activity resulting in preferential hydrolysis of DPPC, DPPE, and DPPG. Incubation of PlcA and PlcB with L. pneumophila culture supernatant activates PLC activity. a, uninduced (−) and isopropyl 1-thio-β-d-galactopyranoside-induced (+) E. coli BL21 cell lysates expressing plcC (BL21(pMS2)) harboring an N-terminal His6 tag or buffer control (Tris-HCl) was incubated with 13.4 mm DPPC, DPPC supplemented with 10 mm ZnCl2, and DPPC supplemented with 10 mm EDTA for 20 h at 37 °C. Lipids subsequently were extracted and subjected to TLC analysis. b, lysate (L), column flow-through (FT), and eluted partially purified Strep-PlcC (E; 400 ng protein) were applied to reducing SDS-PAGE and stained with Coomassie Blue. c, 2 μg of partially purified Strep-PlcC was quantitatively assessed for PC-PLC activity compared with the positive control, 5 milliunits of B. cereus PC-PLC. Further PlcC incubations in parallel were supplemented with 0.1 mm ZnCl2 or 1 mm EDTA and incubated at 37 °C. d, cell lysates of the above mentioned isopropyl 1-thio-β-d-galactopyranoside-induced E. coli BL21 plcC expression clone were incubated with DPPC (PC), DPPE (PE), DPPG (PG), DPPS (PS), PI, SM, and CL for 20 h at 37 °C and analyzed as described in “Experimental Procedures.” All substrates were supplemented with 0.1 mm ZnCl2. e, recombinantly expressed and partially purified Strep-PlcA and Strep-PlcB (about 5 μg of protein) were incubated with DPPG for 5 h at 37 °C with or without the addition of L. pneumophila plcABC mutant culture supernatant and 0.1 mm ZnCl2. All results are representative of at least two additional experiments. St, standard; b, buffer; A, PlcA; B, PlcB.