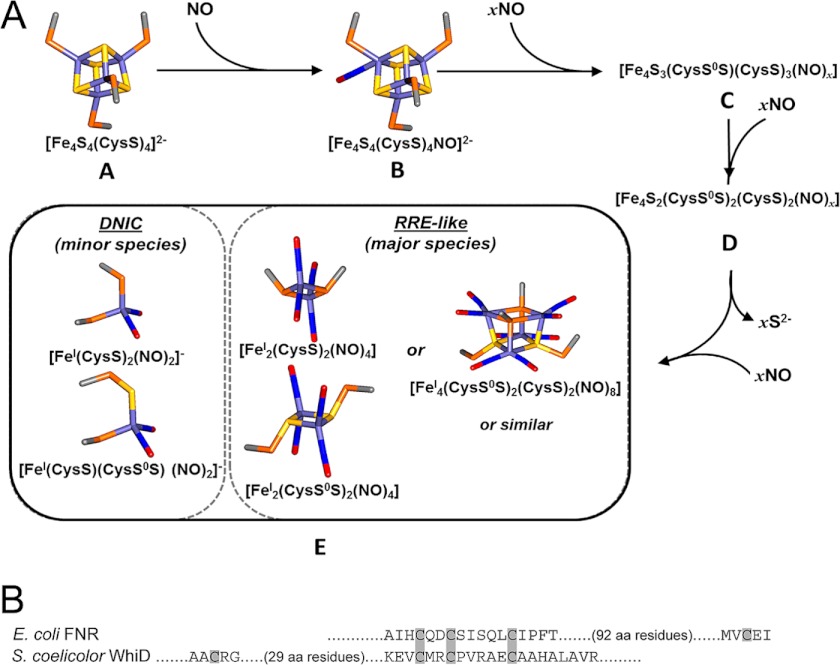
FIGURE 8.
A conserved mechanism for [4Fe-4S] cluster nitrosylation. A, a scheme illustrating proposed steps in the nitrosylation of [4Fe-4S] FNR. The first step of the reaction, A → B, is proposed to correspond to the binding of one NO molecule to the FNR [4Fe-4S] cluster to yield a mono-nitrosylated cluster, which may facilitate further NO binding in steps B → C and C → D. Intermediate D may be the stable, but EPR-silent, tetra-nitrosylated multi-iron species observed during optical titrations (Fig. 2). The last step of the reaction, D → E, results in further nitrosylation of species D to give a product resembling a pair of EPR-silent RRE-like species (∼96% of starting iron) and S = ½ DNIC species (total ∼4%). Models were made using Discovery Studio (Accelrys Software Inc., San Diego, CA) showing iron (pale blue), sulfide (yellow), Cys β-carbon (gray), Cys γ-sulfur (orange), nitrogen (blue), and oxygen (red). B, comparison of amino acid sequence in the cluster-coordinating regions of FNR and WhiD. Note that the spacing between the three coordinating Cys residues is conserved but the identity of the spacing amino acid residues themselves are not. Furthermore, the location of the fourth coordinating Cys residue, although necessarily close to the other three Cys residues in three-dimensional space, is located toward the N terminus in WhiD but is toward the C terminus in FNR.