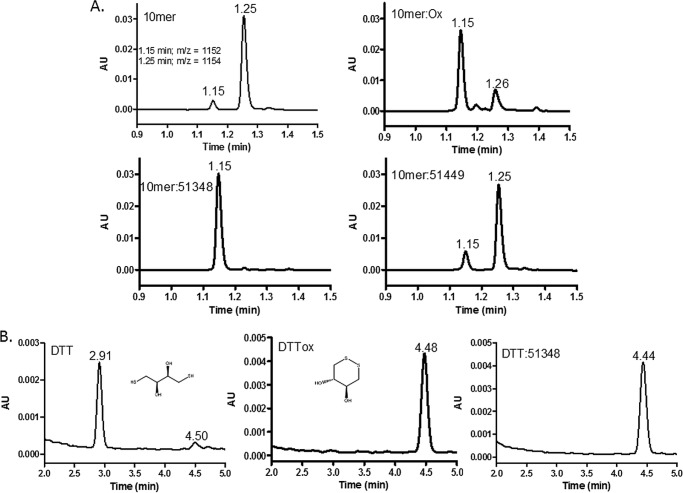
FIGURE 5.
ATPZs facilitate disulfide formation in thiol-containing molecules other than Tau. A, a 10-mer peptide containing two cysteine residues was incubated for 0.5 h at 37 °C in the presence or absence of CNDR-51348 or CNDR-51449, and the mixtures were subsequently analyzed by reversed-phase HPLC-MS. In addition, a pre-oxidized sample of the 10-mer was subjected to HPLC-MS analysis. Peaks with molecular masses corresponding to a peptide with oxidized cysteines and reduced cysteines eluted at 1.15 and 1.25 min, respectively. B, DTT was incubated in the absence (DTT) or presence of CNDR-51348 (DTT:51348) or was pre-oxidized (DTTox) before HPLC-MS analysis. The peaks eluting at 2.91 and 4.44–4.48 min have molecular masses corresponding to the reduced and oxidized forms of DTT, respectively, whose structures are depicted.