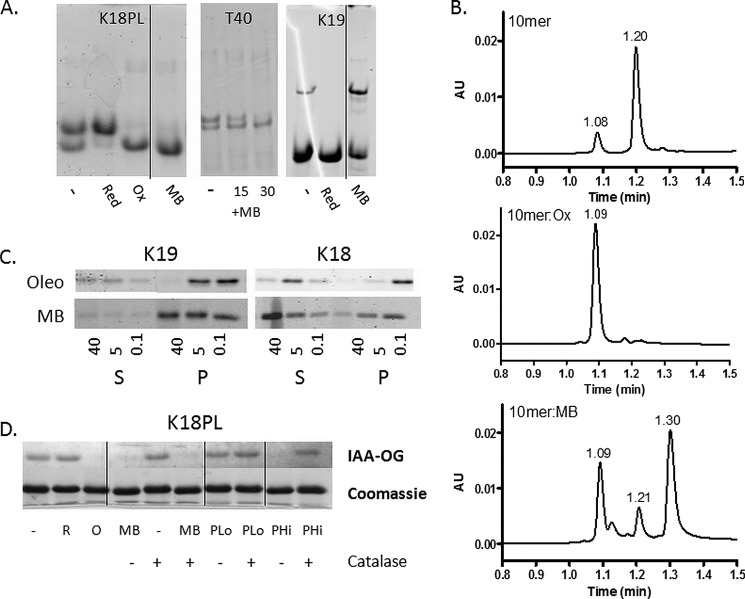
FIGURE 8.
MB induces disulfide formation in molecules other than Tau and does not inhibit 3-R Tau fibrillization. A, K18PL or K19 were incubated for 1 h at 37 °C in the absence (−) of presence of MB (MB) or were pre-reduced (Red) or pre-oxidized (Ox). The samples were then analyzed by native gel electrophoresis. In addition, T40 was incubated in the absence (−) or presence of MB (+MB) for 15 and 30 min followed by analysis by native gel electrophoresis. B, a 10-mer peptide containing two cysteine residues was incubated for 1 h at 37 °C in the absence (10-mer) or presence of MB (10-mer:MB) or was pre-oxidized (10-mer:Ox), and the mixtures were subsequently analyzed by reversed-phase HPLC-MS. Peaks with masses corresponding to a peptide with oxidized cysteines (m/z = 1152) and reduced cysteines (m/z = 1154) eluted at 1.08–1.09 and 1.20–1.21 min, respectively. The peak eluting at 1.30 min in the MB-treated sample is reduced methylene blue, and the shoulder on the peak eluting at 1.09 min is azure B, which is a demethylated form of MB that is found as a minor contaminant in MB preparations. C, K19 and K18 fibrillization reactions were incubated with increasing concentrations (in μm) of MB or oleocanthal (Oleo). After completion of the reactions, the samples were subjected to centrifugation to separate fibrillar Tau (pellet fraction (P)) from non-fibrillar Tau (supernatant fraction (S)). Both the pellet and supernatant fractions were analyzed by SDS-PAGE and Coomassie Blue staining. D, K18PL was left untreated (−) or was pre-reduced (R) or pre-oxidized (O). In addition, K18PL was treated with MB or with 20 μm (PLo) or 1 mm (PHi) hydrogen peroxide both in the presence and absence of catalase. The samples subsequently underwent reaction with IAA-OG followed by SDS-PAGE to evaluate the extent of cysteine oxidation. Both the IAA-OG fluorescence and corresponding Coomassie blue staining are shown for each IAA-OG-treated sample. In A and D, the vertical lines designate sites where non-pertinent gel lanes were removed and figures were subsequently spliced.