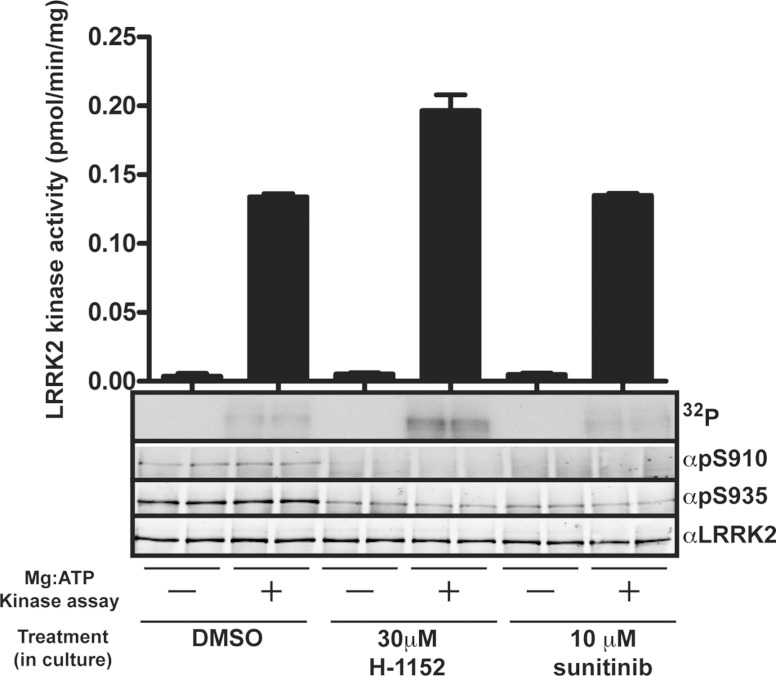
Figure 5. Evidence that Ser910/Ser935 phosphorylation is not mediated by autophosphorylation.
Endogenous LRRK2 was immunoprecipitated from Swiss 3T3 cells treated with DMSO, 30 μM H-1152 or 10 μM sunitinib for 2 h to induce dephosphorylation of Ser910 and Ser935. Immunoprecipitates were washed multiple times with lysis buffer containing 0.5 M NaCl to remove inhibitor and were then incubated in kinase buffer containing 20 μM Nictide in the presence or absence of magnesium ATP (Mg:ATP) for 30 min. Following incubation, immunoprecipitates were centrifuged at 6000 g for 0.5 min and the supernatant was spotted on to P81 paper for measurement of LRRK2 kinase activity. Sample buffer was added to the pelleted beads and LRRK2 Ser910 and Ser935 phosphorylation was quantified following immunoblot analysis with the indicated antibodies. A membrane was also subjected to autoradiography to assess LRRK2 autophosphorylation (32P). The minor effect that H-1152 had on LRRK2 kinase assay is not significant.