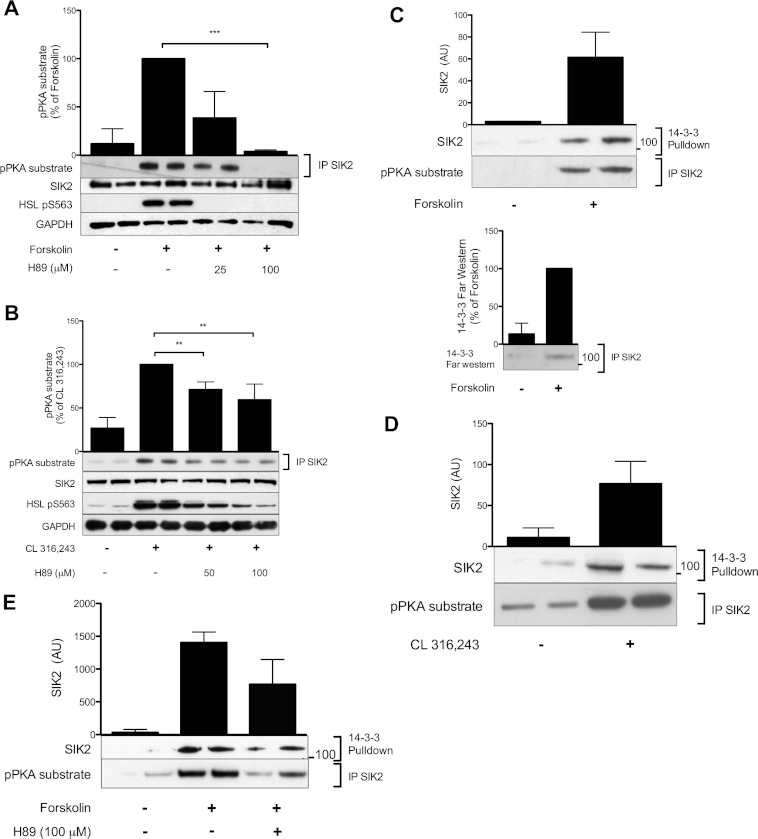
Figure 3. cAMP-induced phosphorylation of SIK2 coincides with 14-3-3 binding: requirement of PKA.
(A and B) The requirement of PKA for the phosphorylation of SIK2 was investigated in 3T3-L1 (A) and primary rat (B) adipocytes. Cells were pre-incubated for 20 min with different doses of the PKA inhibitor H89, followed by stimulation with forskolin (50 μM, 15 min) or CL 316,243 (100 nM, 30 min). Lysates were analysed with regard to total SIK2 and phosphorylation by PKA (pPKA substrate antibodies). HSL phosphoSer563 was used as a control. Quantified phosphorylation data are presented as means±S.D. for three to five individual experiments. **P < 0.01 and ***P < 0.001. 3T3-L1 (C) or rat (D) adipocytes were stimulated with forskolin (50 μM, 15 min) or CL 316,243 (100 nM, 30 min). 14-3-3 binding to SIK2 was demonstrated by the presence of SIK2 in 14-3-3 pull downs using GST–14-3-3ζ coupled to glutathione–Sepharose (C, upper panel and D), or by the direct interaction of recombinant yeast 14-3-3 (BMH1/BMH2) to immunoprecipitated SIK2 on a far-Western blot (C, lower panel). (E) Primary rat adipocytes were pre-incubated for 20 min with the PKA inhibitor H89, followed by stimulation with forskolin (50 μM, 15 min). The binding of 14-3-3 to SIK2 was demonstrated by the presence of SIK2 in 14-3-3 pull downs. Quantified 14-3-3-binding data from pull downs and far-Western blots are presented as means±S.D. for duplicate or single samples and are representative for two individual experiments. The blots shown are representative.