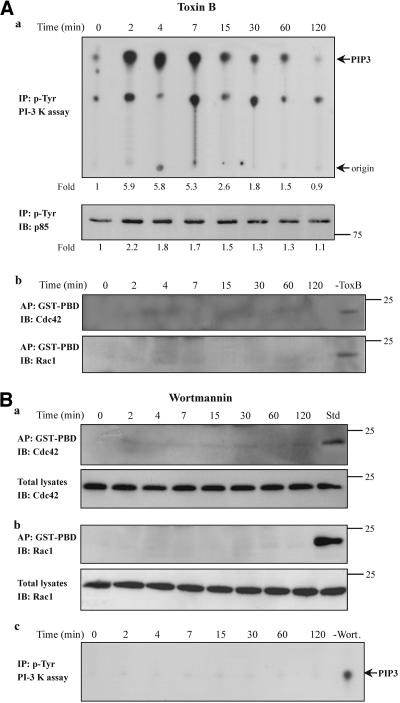
Figure 5.
PI-3 kinase is activated upstream of the small GTPases Cdc42/Rac1 in TNF-α-treated cells. (A) Cells were pretreated with toxin B (50 ng/ml, 2 h) and then incubated with TNF-α (10 ng/ml) for the indicated times. (a) Equal amount of proteins of cell lysates were immunoprecipitated with an anti-phosphotyrosine antibody and subjected to an in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, by using PIP2 as substrate. The number below each lane indicates the fold amount of PIP3 product, with that of untreated cells taken as 1 (top). The phosphorylation of the p85 regulatory subunit of PI-3 kinase that was immunoprecipitated in the kinase assay was assessed by immunoprecipitation (IP) with an anti-phosphotyrosine antibody and immunoblotting (IB) with anti-PI-3 kinase (p85) antibody. The number below each lane indicates the fold phosphorylation of p85, with that of untreated cells taken as 1 (bottom). (b) The activated forms of Cdc42 (top) or Rac1 (bottom) in the presence or absence (-ToxB) of toxin B was determined by affinity precipitation (AP) with GST-PBD and then by IB with the respective antibodies. (B) Cells were pretreated with wortmannin (100 nM, 30 min) and then with TNF-α. Equal volume of cell lysates from untreated or TNF-α-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-agarose beads. Precipitated GTP-Cdc42 (a, top) or GTP-Rac1 (b, top) was detected by immunoblot (IB) with anti-Cdc42 or anti-Rac1 antibody, respectively. Equal volumes of total lysates from untreated and TNF-α-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and IB with monoclonal anti-Cdc42 (a, bottom) or anti-Rac1 (a, bottom) antibody, respectively. (c) The lipid kinase activity of PI-3 kinase in the presence or absence (-Wort.) of wortmannin was determined by the in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, by using PIP2 as substrate. Results shown are representative of three similar experiments. PIP3, phosphatidylinositol-3,4,5-trisphosphate.