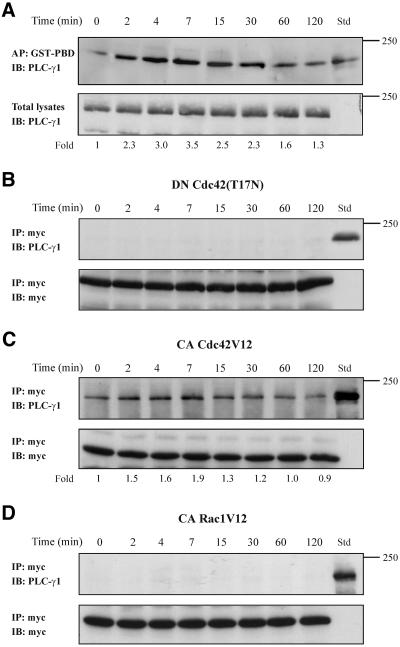
Figure 8.
PLC-γ1 associates in vivo with Cdc42. (A) Equal volumes of cell lysates from untreated or TNF-α-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-agarose beads. Coprecipitated PLC-γ1 was detected by immunoblot (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). Equal volume of total lysates from untreated and TNF-α-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with anti-PLC-γ1 antibody (bottom). The number below each lane indicates the normalized fold amount of PLC-γ1 precipitated with GST-PBD, with that of untreated cells taken as 1. (B) Cells were transfected with the inactive Cdc42(T17N) and then stimulated with TNF-α for the indicated times. Myc-tagged Cdc42(T17N) was immunoprecipitated (IP) with mouse monoclonal anti-myc epitope antibody, and Western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Cdc42(T17N) in the immunoprecipitates (bottom). (C) Cells were transfected with the constitutively active Cdc42V12 and then stimulated with TNF-α for the indicated times. Myc-tagged Cdc42V12 was IP with mouse monoclonal anti-myc epitope antibody and western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Cdc42V12 in the immunoprecipitates (bottom). The number below each lane indicates the fold amount of PLC-γ1 coimmunoprecipitated with Cdc42V12, with that of untreated cells taken as 1. (D) Cells were transfected with the constitutively active Rac1V12 and then stimulated with TNF-α for the indicated times. Myc-tagged Rac1V12 was IP with mouse monoclonal anti-myc epitope antibody and Western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Rac1V12 in the immunoprecipitates (bottom). The experiments were repeated four times with similar results.